Elecsys® PIVKA-II
A sensitive and accurate tool for use as an aid in the diagnosis of HCC
What are the current limitations in current HCC surveillance methods?1

Only 63% Sensitivity2
of Ultrasound + AFP
in detecting early stage HCC
37%
will be missed

- Poor performance in patients with fibrotic changes and fatty infiltration of the liver
- Difficult to perform in obese patients
- Difficult to detect small lesions (< 2cm)
- Limited capacity in public hospitals and rural settings
- Operator variability

AFP is not specific for HCC. It can be elevated (false positive) in the following conditions:
- Cirrhosis
- Active hepatitis
- Other types of tumours
AFP can be normal (false negative) in the following conditions:
- Certain HCC patients have normal AFP throughout the entire disease course
- Small size HCC (tumour < 2 cm)
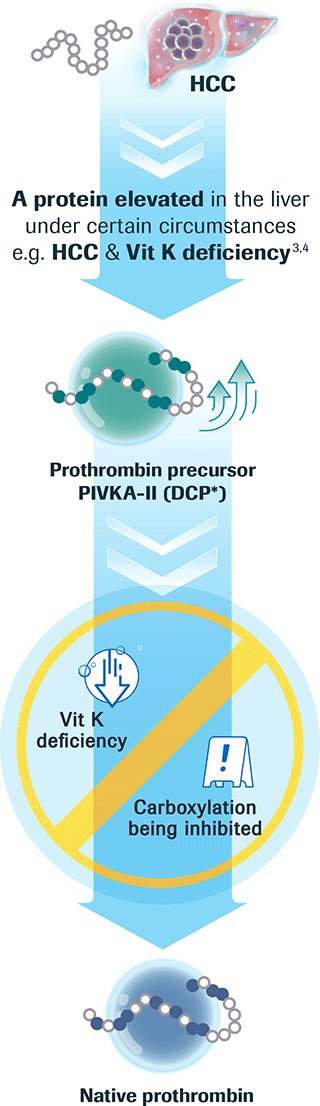
What is PIVKA-II?
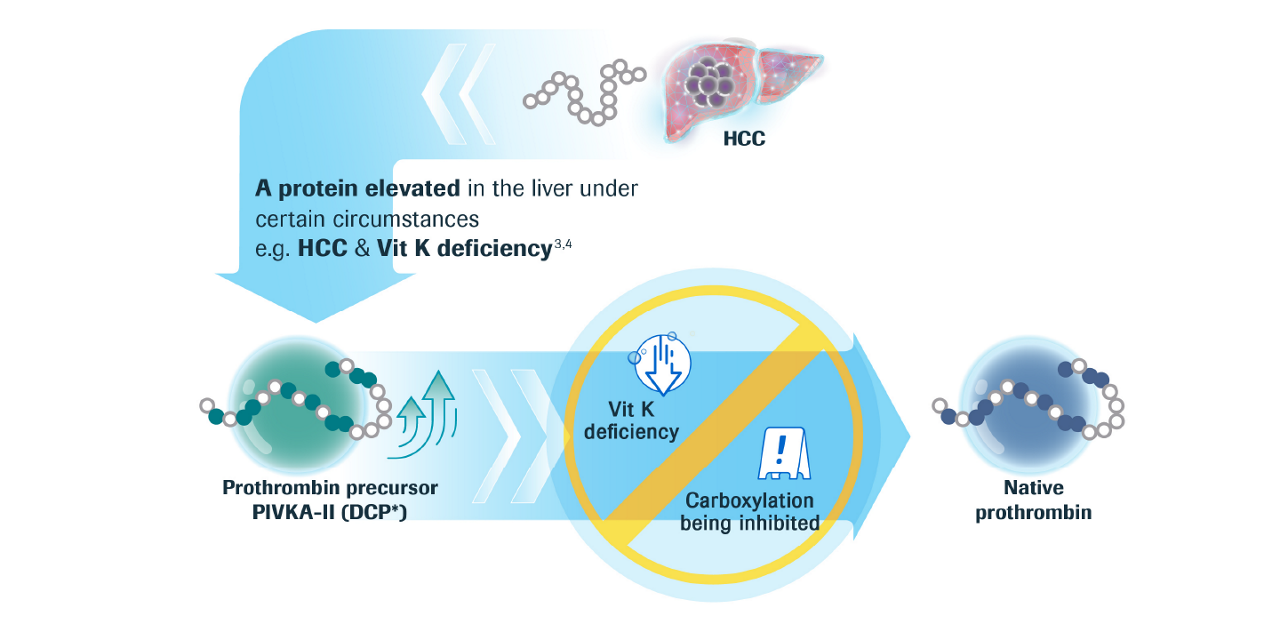
PIVKA-II detects HCC with a higher sensitivity vs AFP5
Comparison between PIVKA-II and AFP
| All HCC | Early Stage HCC* | Late Stage HCC† | ||||
|---|---|---|---|---|---|---|
| Marker | PIVKA-II | AFP | PIVKA-II | AFP | PIVKA-II | AFP |
| Sensitivity (95% CI) |
86.90%(80.8%, 91.6%) |
51.80% (44%, 59.5%) |
77.90%(67%, 86.6%) |
36.40% (25.7%, 48.1%) |
94.50%(87.6%, 98.2%) |
64.80% (54.1%, 74.6%) |
Specificity |
83.70% (77.9%, 88.4%) |
98.10% (95.1%, 99.5%) |
83.70% (77.9%, 88.4%) |
98.10% (95.1%, 99.5%) |
83.70% (77.9%, 88.4%) |
98.10% (95.1%, 99.5%) |
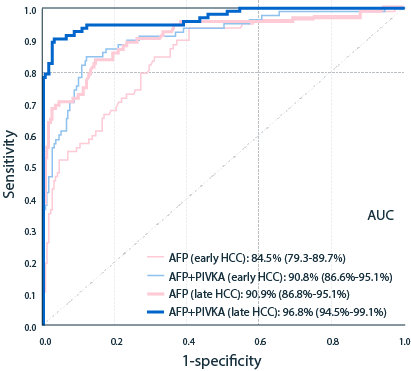
| ROC AUC§ | 90.80% (87.5%−94.1%) |
88% (84.5%−91.5% |
84.70% (78.7%−90.8%) |
84.50% (79.3%−89.7%) |
95.50% (93.2%−98.7%) |
90.90% (86.8%−95.1%) |
| All HCC | ||
|---|---|---|
| Marker | PIVKA-II | AFP |
| Sensitivity (95% CI) |
86.90%(80.8%, 91.6%) |
51.80% (44%, 59.5%) |
| Specificity (95% CI) |
83.70% (77.9%, 88.4%) |
98.10% (95.1%, 99.5%) |
| ROC AUC§ | 90.80% (87.5%−94.1%) |
88% (84.5%−91.5%) |
| Early Stage HCC* | ||
|---|---|---|
| Marker | PIVKA-II | AFP |
| Sensitivity (95% CI) |
77.90%(67%, 86.6%) |
36.40% (25.7%, 48.1%) |
| Specificity (95% CI) |
83.70% (77.9%, 88.4%) |
98.10% (95.1%, 99.5%) |
| ROC AUC§ | 84.70% (78.7%−90.8%) |
84.50% (79.3%−89.7%) |
| Late Stage HCC† | ||
|---|---|---|
| Marker | PIVKA-II | AFP |
| Sensitivity (95% CI) |
94.50%(87.6%, 98.2%) |
64.80% (54.1%, 74.6%) |
| Specificity (95% CI) |
83.70% (77.9%, 88.4%) |
98.10% (95.1%, 99.5%) |
| ROC AUC§ | 95.50% (93.2%−98.7%) |
90.90% (86.8%−95.1%) |
At the cut-off of 28.4 ng/mL Elecsys PIVKA-II shows a higher sensitivity
vs AFP surveillance cut-off of 20 ng/mL5,6
*BCLC stages 0, A † BCLC stages B,C,D ‡ Applies to sensitivity and specificity only § Area under the Curve
What is the best recommended approach for better and more accurate diagnosis?
The combination of PIVKA-II and AFP
has markedly better sensitivity for detecting HCC vs AFP alone7
Comparison between PIVKA-II + AFP and AFP
| AFP | AFP + PIVKA-II | |
|---|---|---|
| Sensitivity (All HCC) |
51.8% | 91.7% |
| Sensitivity (Early Stage HCC)* |
36.4% | 87.0% |
| Specificity (Late Stage HCC)† |
64.8% | 95.6% |
| Specificity | 98.1% | 82.2% |
* BCLC stages 0, A; † BCLC stages B,C,D

| AFP | AFP + PIVKA-II | |
|---|---|---|
| Sensitivity (All HCC) |
51.8% | 91.7% |
| Sensitivity (Early Stage HCC)* |
36.4% | 87.0% |
| Specificity (Late Stage HCC)† |
64.8% | 95.6% |
| Specificity | 98.1% | 82.2% |
* BCLC stages 0, A; † BCLC stages B,C,D
By using the combination of PIVKA-II, AFP and US, we can improves the sensitivity for detection of HCC

By using the combination of PIVKA-II, AFP and US, we can improves the sensitivity for detection of HCC
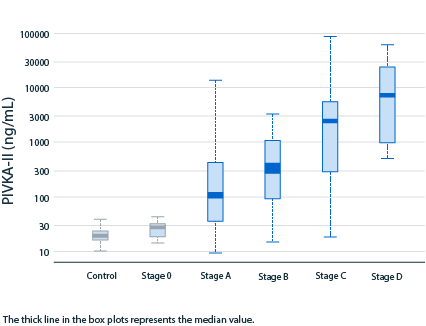
PIVKA-II concentration reliably shows gradual
HCC disease progression and clear differentiation5,7
Range of PIVKA-II distribution
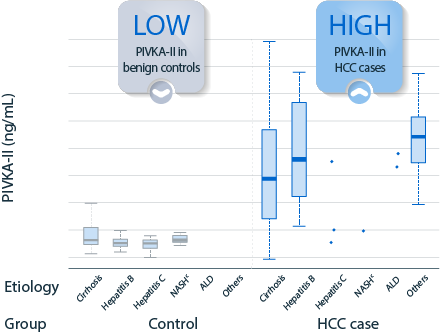
PIVKA-II concentration and disease etiology
References
- Simmons,O.etal.(2017),Predictorsofadequateultrasoundqualityforhepatocellularcarcinomasurveillanceinpatientswithcirrhosis.AlimentPharmacolTher,45:169-177.doi:10.1111/apt.13841; EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma., Journal of Hepatology, Volume 69, Issue 1, 2018, Pages 182-236, ISSN 0168-8278; Sherman M. Limitations of screening for hepatocellular carcinoma. Hepat Oncol. 2014;1(2):161–163. doi:10.2217/hep.13.22
- Singal, A.G.,et al.. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology. 2018 May;154(6):1706-1718.e1.
- Liebmann, H.A. et al. (1984). Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Eng J Med 310, 1427-1431.
- Ono, M. et al. (1990). Measurement of immunoreactive prothrombin precursor and vitamin-K-dependent gamma-carboxylation in human hepatocellular carcinoma tissues: Decreased carboxylation of prothrombin precursor as a cause of des-gamma-carboxy prothrombin synthesis. Tumour Biol 11, 319-326.
- Chan, H. L. Y., Vogel, A., Berg, T., De Toni, E. N., Kudo, M., Trojan, J., ... & Piratvisuth, T. (2020, November). ELECSYS PIVKA-II AND ELECSYS AFP ASSAYS DEMONSTRATE GOOD CLINICAL PERFORMANCE FOR HEPATOCELLULAR CARCINOMA (HCC) DIAGNOSIS ACROSS DIFFERENT DISEASE STAGES AND ETIOLOGIES. In The Liver Meeting Digital Experience™. AASLD.
- Chang TS et al. Am J Gastroenterol 2015;110:836–844
- Roche CE method sheet PIVKA-II 2020 (Roche studies No. RD002542 and RD002543)
MAP-2023-JUL-002