Your assay choice matters
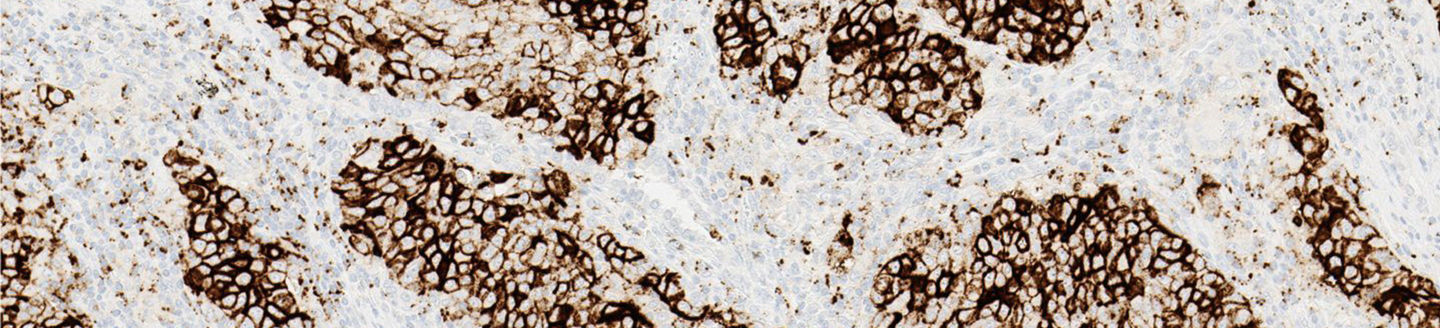
Using the right test to determine PD-L1 status for immunotherapy options is important, and the VENTANA PD-L1 (SP142) Assay is an FDA-approved test for TECENTRIQ®. This innovative assay is the first to evaluate patient PD-L1 expression using both tumor cell (TC) and tumor-infiltrating immune cell (IC) staining. Determining a patient's PD-L1 expression level can give insight to the overall survival that may be achieved from TECENTRIQ®.
The VENTANA PD-L1 (SP142) Assay:
- FDA approved to identify NSCLC patient treatment benefit from TECENTRIQ®
- Novel scoring algorithm using PD-L1 staining in both TC and IC
- Designed to enhance visual contrast of immune cell staining within the tumor microenvironment
The PD-L1 (SP142) Assay gives you the confidence to guide immunotherapy decisions in NSCLC.
*All randomized patients in a NSCLC phase Ill study observed benefit from TECENTRIQ® regardless of PD-L1 status.