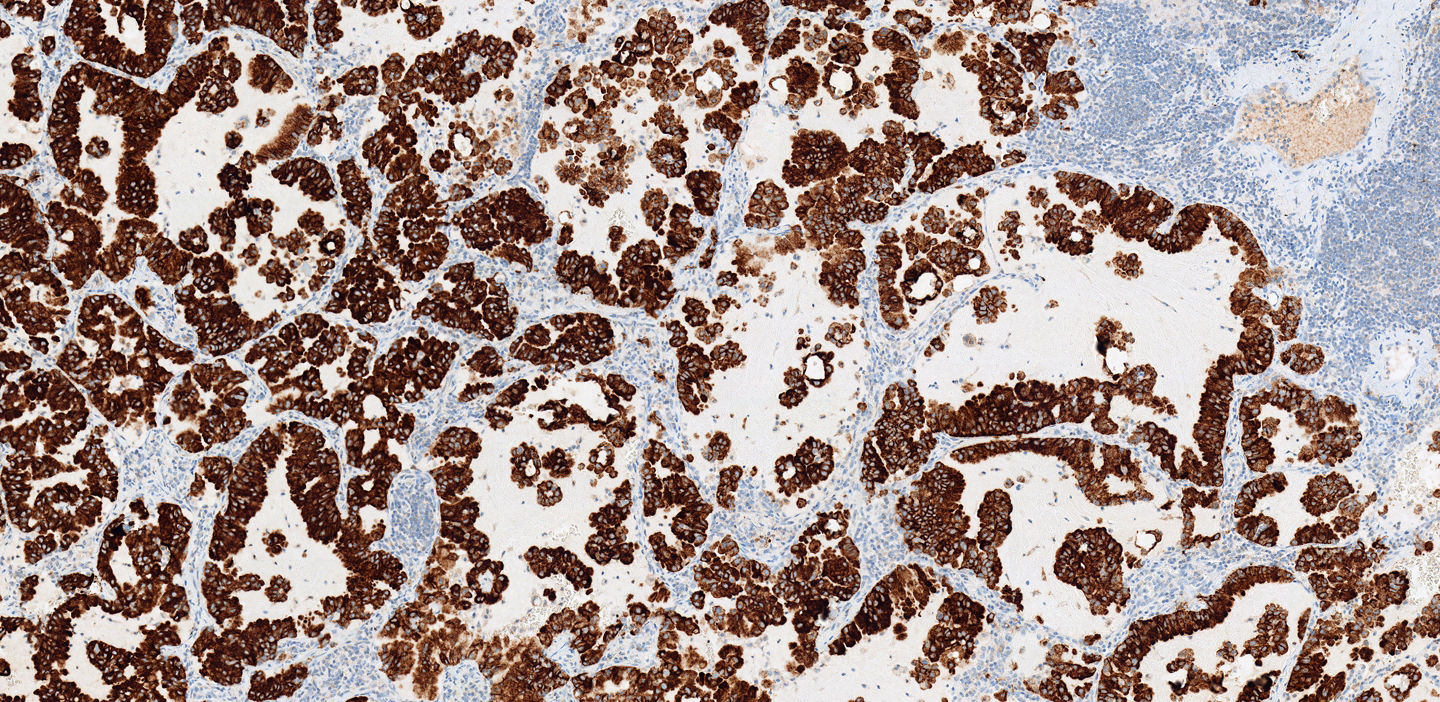
Les directives cliniques recommandent l’analyse de routine des mutations génétiques pour tous les adénocarcinomes, y compris le réarrangement génétique ALK-EML4. Des tests correspondants doivent être réalisés le plus rapidement possible après l’examen histologique, et dans tous les cas, avant l’instauration d’un traitement ciblé pour le patient. La pratique actuelle en matière de test comprend l’IHC et la FISH.
Anticorps primaire monoclonal de lapin VENTANA anti-ALK (D5F3) (DIV CE)

Traitement ciblé des patients ALK positifs
- L’anticorps primaire monoclonal de lapin VENTANA anti-ALK (D5F3)*, coloré avec le kit de détection OptiView IHC DAB et l’amplification OptiView, reconnaît la structure cible du traitement, la protéine ALK.
- Les directives cliniques recommandent un temps de traitement rapide pour permettre une instauration précoce de thérapies ciblées
- L’ALK a une sensibilité et une spécificité comparables à celles de l’hybridation in situ en fluorescence (FISH)
- Grâce au test VENTANA anti-ALK (D5F3), des décisions thérapeutiques peuvent être prises plus rapidement pour les patients atteints de CPNPC avancé
* Test VENTANA ALK (D5F3)
Cancer du poumon non à petites cellules (CPNPC)
XALKORI® (crizotinib), ZYKADIA® et ALECENSA® (alectinib) portent le marquage CE et sont cliniquement efficaces pour le traitement des patients atteints de CPNPC métastatique, dont les tumeurs ont été testées ALK positives en utilisant une procédure de test de l’ALK autorisée par la FDA.1, 3, 7
- XALKORI (crizotinib) est indiqué pour le traitement des patients atteints de CPNPC métastatique positif pour l’ALK¹
- ZYKADIA (céritinib) est indiqué pour le traitement des patients atteints de CPNPC ALK positif métastatique qui n’ont pas encore été traités ou chez qui une progression est survenue sous crizotinib ou qui n’ont pas toléré le crizotinib3
- ALECENSA (alectinib) est indiqué pour le traitement des patients atteints de CPNPC ALK positif métastatique qui n’ont pas encore été traités ou chez qui une progression est survenue sous crizotinib ou qui n’ont pas toléré le crizotinib7
Avantages techniques du test par IHC
Avec le test ALK FISH, l’évaluation des résultats des patients est techniquement difficile et il existe la possibilité de faux négatifs. Des études actuelles montrent que le test VENTANA ALK (D5F3) coloré avec le kit de détection et l’amplification OptiView IHC DAB est sensible et spécifique pour la détermination du statut ALK et est donc préférable à l’alternative du test ALK FISH. Dans le cadre de plusieurs évaluations, des patients ALK positifs par IHC et négatifs par FISH tirant des bénéfices d’un traitement par XALKORI, ZYKADIA ou ALECENSA ont pu être identifiés. 8,9,10,11
Test et détection avec amplification de VENTANA ALK (D5F3) vs FISH
Afficher le tableau completTest et détection avec amplification de VENTANA ALK (D5F3) vs FISH
Dans une étude, van der Wekken et al. sont arrivés à la conclusion que «...l’ALK IHC dichotomique est supérieure à l’ALK FISH pour les petites biopsies et les biopsies à l’aiguille fine (BAF) afin de prédire la réponse tumorale et la survie avec le crizotinib chez les patients atteints de CPNPC avancé.» 7
Dans une étude, van der Wekken et al. sont arrivés à la conclusion que «...l’ALK IHC dichotomique est supérieure à l’ALK FISH pour les petites biopsies et les biopsies à l’aiguille fine (BAF) afin de prédire la réponse tumorale et la survie avec le crizotinib chez les patients atteints de CPNPC avancé.» 7
| Test VENTANA ALK (D5F3) avec détection et amplification OptiView DAB | ALK FISH | |
|---|---|---|
Facile à noter |
|
|
| Temps de traitement plus rapide |
|
|
| Coloration en champ clair vs coloration en fluorescence |
|
|
Références
1. XALKORI® (crizotinib) [notice d’emballage], New York, NY: Pfizer; 2012.
2. Ferlay, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D., Bray, F. (2012). GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr (last accessed March 2016).
3. ZYKADIA (céritinib) [notice d’emballage], Whippany, NJ: Novartis Pharmaceuticals Corporation 2016.
4. ALECENSA (alectinib) [notice d’emballage], San Francisco, CA: Genentech; 2017.
5. World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Lyon, France http://globocan.iarc.fr/ Default.aspx. Accessed August 1, 2014.
6. Lung Cancer Survival Rates and Prognosis. National Cancer Institute at the National Institutes of Health. Bethesda, MD. http://www.cancer.gov/cancertopics/types/lung/cancer-survival-prognosis Accessed August 1, 2014.
7. van der Wekken et al Dichotomous ALK-IHC is a better predictor for ALK inhibition outcome than traditional ALK-FISH in advanced non-small cell lung cancer, Clinical Cancer Research, 2017. Available at http://clincancerres.aacrjournals.org/content/early/2017/02/09/1078-0432. CCR-16-1631.
8. Zhou J, Zhao J, Sun K, Wang B, Wang L, et al. Accurate and Economical Detection of ALK Positive Lung Adenocarcinoma with Semiquantitative Immunohistochemical Screening. PLoS ONE (2014) 9(3): e92828. doi:10.1371/journal.pone.0092828.
9. Ling Shan, Fang Lian, Lei Guo, Xin Yang, Jianming Ying and Dongmei Lin. Combination of conventional immunohistochemistry and qRT-PCR to detect ALK rearrangement. Diagnostic Pathology 2014, 9:3. doi:10.1186/1746-1596-9-3.
10. Ying, J.; Guo, L.; Qiu, T.; Shan, L.; Ling, Y.; Liu, X.; Lu, N. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Annals of Oncology. 24(10):2589-2593, October 2 Modern Pathology (2013) 26, 1545–1553; doi:10.1038/modpathol.2013.87; published online 7 June 2013.
11. Mok T, Peters S, Camidge DR. Patients with ALK IHC-positive/fish-negative NSCLC benefit from ALK TKI treatment: response data from the global ALEX trial. Presented at: the IASLC 18th World Conference on Lung Cancer; October 15-18; Yokohama, Japan. Poster MA 07.01
L’anticorps primaire monoclonal de lapin VENTANA anti-ALK (D5F3) (VENTANA anti-ALK (D5F3)) est destiné à la détection de la protéine kinase lymphome anaplasique (ALK) dans des tissus fixés au formol et inclus dans la paraffine de cancer du poumon non à petites cellules (CPNPC), qui ont été colorés en utilisant des appareils BenchMark IHC/ISH. Il est indiqué comme aide lors de l’identification des patients éligibles à un traitement par XALKORI® (crizotinib), ZYKADIA® (céritinib) ou ALECENSA® (alectinib).
Les résultats de ce produit doivent être évalués par un pathologiste qualifié en tenant compte de l’examen histologique, des informations cliniques pertinentes et des contrôles appropriés.
Ce produit est destiné à une utilisation dans le cadre du diagnostic in vitro (DIV).