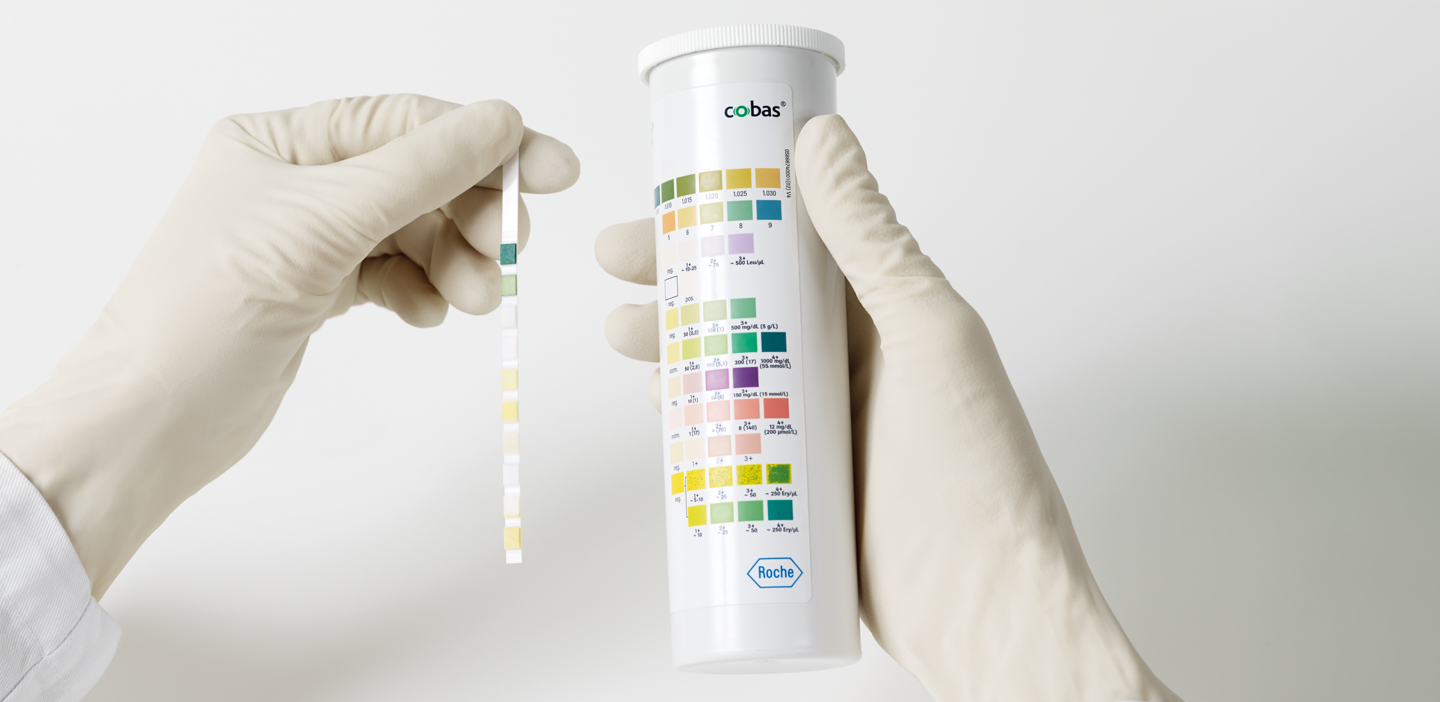
Urinalysis was the first laboratory test performed in medicine and has been used for several thousand years.1 Urinalysis can reveal serious damage on kidney diseases that are asymptomatic in early stages yet treatable when identified in time, enabling earlier and potentially more convenient treatment of patients than testing blood samples.2
Today Roche offers a broad portfolio of urinalysis solutions for different customer needs. Drawing on our over 50 years of experience in urinalysis, starting with the launch of the first Combur-Test® strip, we have continuously improved strip technology for clinical and general practice. In response to customer needs for increased efficiency and safety, we have developed a range of analyzers with differing degrees of automation and throughput capabilities.