For localized information and support, would you like to switch to your country-specific website for {0}?
Confidence starts with clarity
Harness the power of artificial intelligence (AI) to remove time-consuming tasks from your pathology workflow and allow your team to focus on more meaningful tasks.
Pioneers in digital pathology, Roche was the first company to offer a comprehensive portfolio of FDA-cleared image analysis algorithms and digital read applications for the five key immunohistochemistry (IHC) breast markers.1,2 We have since evolved our technology, launching whole slide pathology image analysis algorithms.
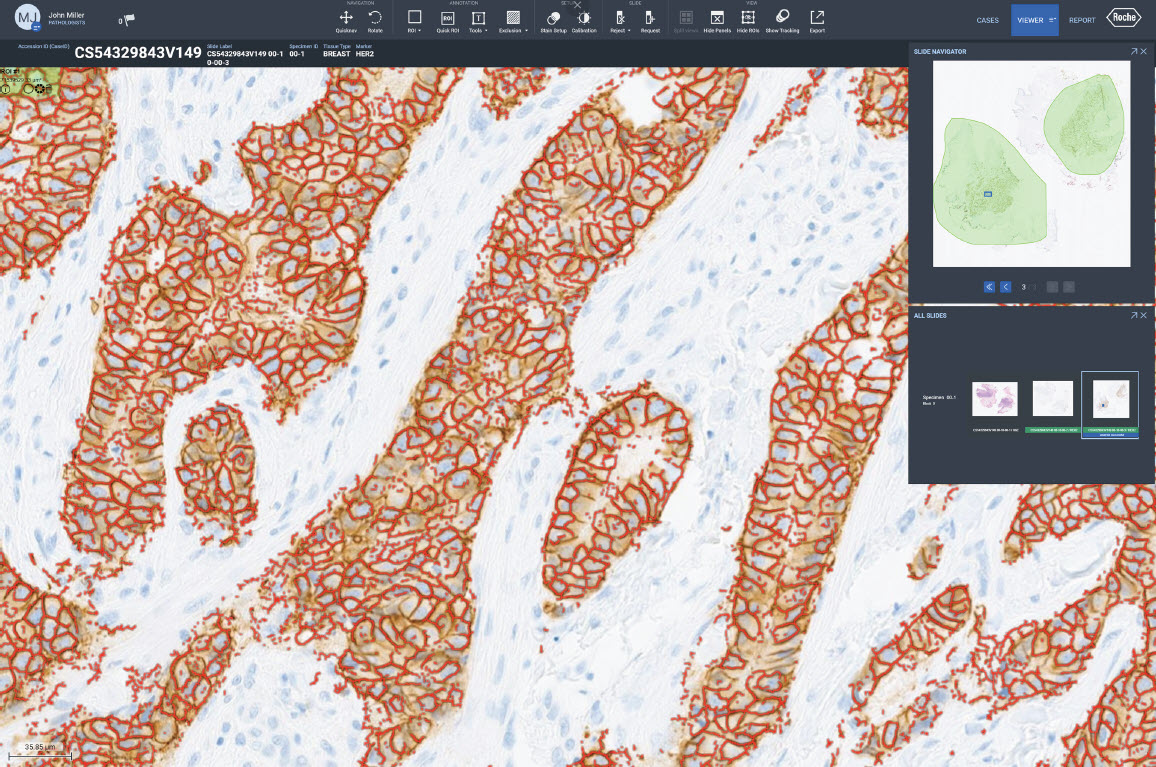
Image analysis algorithms for pathology decision support, provide analysis of VENTANA® slide scanner images stained with a Roche Tissue Diagnostics assay. The algorithms extract meaningful information from pathology slide images, so pathologists can quickly, accurately, and confidently assess whole tissue slide images.
Our integrated and ready-to-use digital pathology algorithms include:
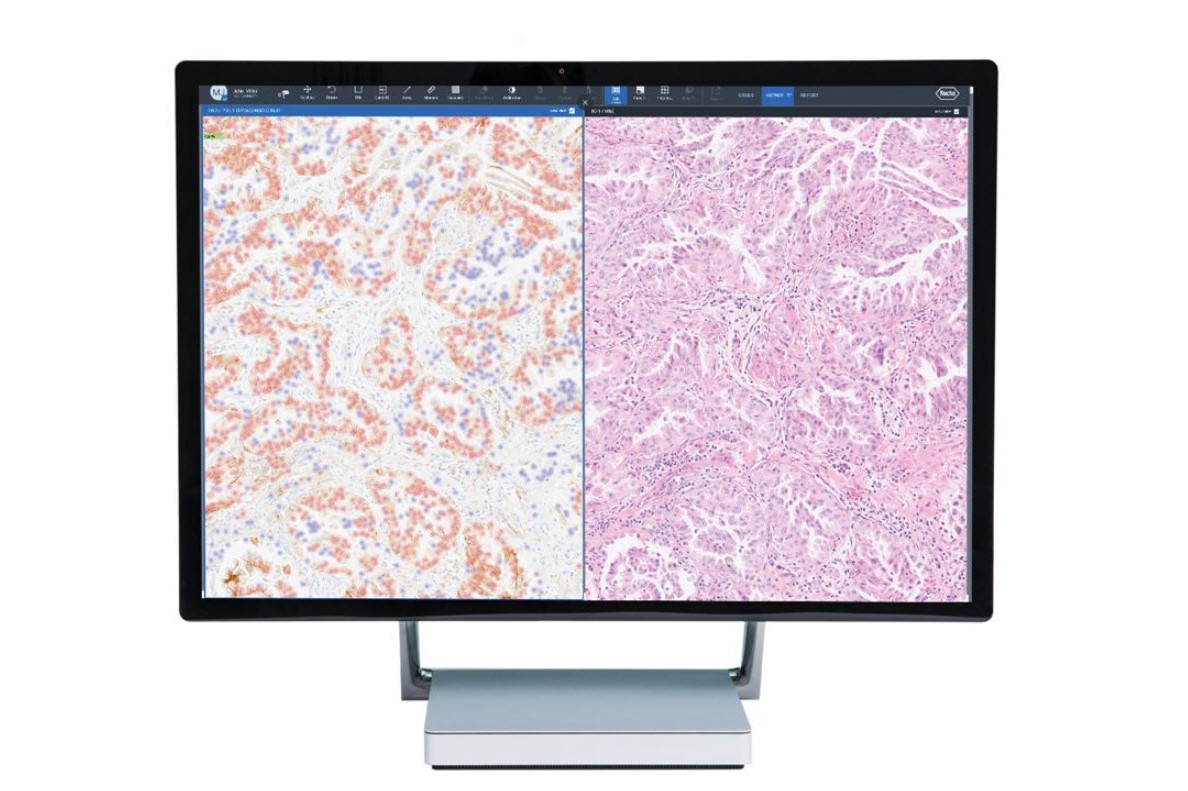
- uPath HER2 (4B5) image analysis, Breast*
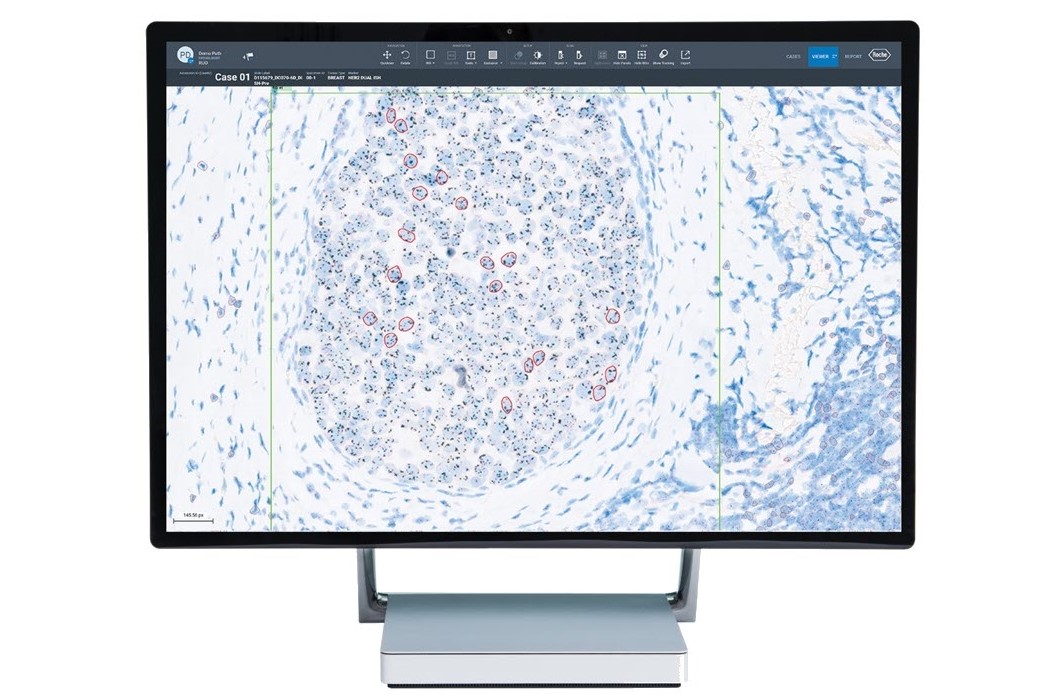
- uPath HER2 Dual ISH image analysis, Breast*
- uPath PD-L1 (SP263) image analysis, NSCLC*
- uPath ER (SP1) image analysis, Breast**
- uPath PR (1E2) image analysis, breast**
- uPath Ki-67 (30-9) image analysis, breast**
- PD-L1 (SP142), breast carcinoma algorithm by navify®Digital Pathology**
Advancing precision medicine with an open digital pathology environment and AI partnerships
The Roche Digital Pathology Open Environment allows for the integration of tumor tissue image analysis tools with navify® Digital Pathology, giving pathologists access to a broad set of diagnostics tools from Roche and third-party software developers.
We collaborate with other industry innovators to bring the most advanced solutions to our customers. Roche has partnered with PathAI to develop digital pathology algorithms with AI-enabled interpretation for companion diagnostics, to drive advancement in precision medicine solutions. The integration of Ibex clinical decision support software with the navify platform enhances pathology workflows to aid breast and prostate cancer diagnostics.
Roche will continue to invest in developing next-generation digital pathology algorithms, whilst facilitating an open digital pathology environment to share technology across industry and research partners to advance efficiency and precision in diagnostics.
Featured products
Benefits of image analysis algorithms from Roche
For confident decision making
Our image analysis algorithms generate actionable, objective, and reproducible results. The algorithms are trained using a robust and representative data set to automatically classify cells and provide assay-specific scoring.
According to a study, image analysis algorithms enhance pathologist reporting efficiency, demonstrating a 66.8% reduction in diagnostic time.3 Pathologists can make confident decisions based on quick, accurate analysis.
Integrated and ready-to-use digital pathology algorithms
Fully embedded into navify Digital Pathology enterprise software, Roche image analysis algorithms enable seamless viewing, aligning, syncing, sharing, and reporting. The AI-powered algorithms, streamline processes, removing time-consuming tasks for a more efficient and productive pathology workflow.
Ensuring reproducible results
Roche Digital Pathology in vitro diagnostics (IVD) products are validated on a robust, reliable, and representative set of benchmark data as part of the end-to-end Roche pathology laboratory ecosystem. This validation process removes variables to ensure reproducible results.
Both our clinical algorithms and research-use-only (RUO) image analysis algorithms are validated by a group of pathologists at every stage of development.
Help improve patient outcomes with precision medicine
Enhancing precision medicine for improved personalized healthcare and clinical decision-making for better patient care. Image analysis algorithms have the potential to enhance concordance of challenging cases in breast cancer.3

Partner with us to enhance patient care together
The Roche Digital Pathology Open Environment streamlines the integration of third-party tumor tissue analysis tools with Roche's navify® Digital Pathology enterprise software. If you have an opportunity for collaboration, we would like to hear from you.
Explore more
*CE-IVD marked. For Research Use Only in the US. Not for use in diagnostic procedures.
**For Research Use Only. Not for use in diagnostic procedures.
navify® Digital Pathology enterprise software is CE-IVD marked, in the US: For Research Use Only. Not for use in diagnostic procedures in the US.
Registration Status: CE-IVD marked. In the United States, Roche Digital Pathology Dx (VENTANA DP 200) is available as a whole slide imaging system to aid in primary diagnosis. Refer to the product-specific labeling for the regulatory status of scanners, software and/or algorithms. IVD products can be used for in-vitro diagnostic use. Research Use Only (RUO) products are for research use only and not for diagnostic procedures.
VENTANA and NAVIFY are trademarks of Roche. Other product names and trademarks are the property of their respective owners.
References:
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K111543 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K121516 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K130515 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K111755 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K121033 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K111872 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K142965 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K122143 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K111869 ; https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K140465 ;
- https://www.prnewswire.com/news-releases/ventana-receives-fda-clearance-for-estrogen-receptor-er-image-analysis-and-digital-read-application-for-breast-cancer-234579611.html
- Gough, M. et al. Improved concordance of challenging human epidermal growth factor receptor 2 dual in-situ hybridisation cases with the use of a digital image analysis algorithm in breast cancer. Histopathology. 2023 Jun 23; 83:647-656.