For localized information and support, would you like to switch to your country-specific website for {0}?
Improving heart failure outcomes together at every step
We discover and develop novel, high-quality, accurate assays for heart failure biomarkers.
As the leader in in vitro diagnostics (IVD), we discover and develop novel, high-quality, accurate assays for heart failure diagnostics, through:
- Continuous innovation: Roche pioneered N-terminal pro-BNP (NT-proBNP) and has continued to invest in exploring the clinical utility of this biomarker, resulting in claim extensions that make it the NT-proBNP assay with the highest number of intended uses vs. main IVD providers1-9
- Connecting solutions: At Roche, we are fully committed to supporting the optimal implementation of the biomarkers you need, with innovative solutions to assist healthcare professionals (HCPs) in improving outcomes
- Co-creating evidence: We are proud to offer the most extensively validated NT-proBNP assay, giving you a cost-effective way to provide information to assist in clinical decision-making and treatment modification10,11,12
The impact of heart failure
The burden of cardiovascular diseases is substantial and expected to increase in coming years.13-17 Heart failure alone carries a significant burden.16 More than 64 million people are living with heart failure.16 30% of patients are readmitted, 15% die in the 60–90 days following discharge after acute heart failure, and 50% of them will die within 5 years of the moment they got admitted to the hospital18,44-47
Economic impacts are also severe, with the global economic burden of heart failure estimated at $346 billion USD (expected to increase 127% by 2030). This includes direct medical costs as well as indirect costs related to loss of productivity and caregiving needs.19,20 Imagine the impact that accurate and timely diagnosis of heart failure could have on this huge disease burden. At Roche, we have been working to reduce this burden for over 20 years.2,21-25
Our work has led to extensive clinical validation, including rule-in-rule-out processes in diverse populations, and has informed major clinical guidelines.27,28
The importance of heart failure diagnostics
Diagnosis of acute or chronic heart failure is commonly missed or delayed by years.29-32 The symptoms of heart failure are not specific, which often leads to misdiagnosis.33,34 Early recognition of the risk to develop heart failure with cardiac biomarker NT-proBNP and timely initiation of appropriate treatment can help reduce morbidity and mortality.24
Roche is committed to reducing the burden of heart failure. Especially in patients with type 2 diabetes mellitus, who are more likely to die from cardiovascular diseases than the general population.35
We are working extensively with patient communities, clinical societies and policy makers to combat underdiagnosis and late diagnosis of heart failure patients. For example, the ‘Peptide for Life’ initiative can help to overcome barriers to natriuretic peptide use in the emergency department.36-38
As the pioneer of NT-proBNP, we at Roche support clinical decision-making at every stage of care in heart failure,1-5 with assays that can help provide diagnostic confidence.4,5 We have a history of over 20 years of NT-proBNP evidence generation, with our NT-proBNP assay featured in more than 1,100 peer-reviewed publications, and clinically validated results integrated into clinical practice and guidelines.12,39-42
Featured products
Benefits of Roche diagnostic solutions for managing heart failure
Roche NT-proBNP assists in clinical decision making
Serial measurements of NT-proBNP are a strong predictor of outcomes in heart failure supporting routine NT-proBNP monitoring to assist in clinical decision-making,39 aiding in risk stratification, prognosis during hospitalization and post-discharge prognosis.43
Highly clinically validated assay
We have a history of over 20 years of NT-proBNP evidence generation, with our NT-proBNP assay featured in more than 1,100 peer-reviewed publications, and clinically validated results integrated into clinical practice and guidelines.12,39-42
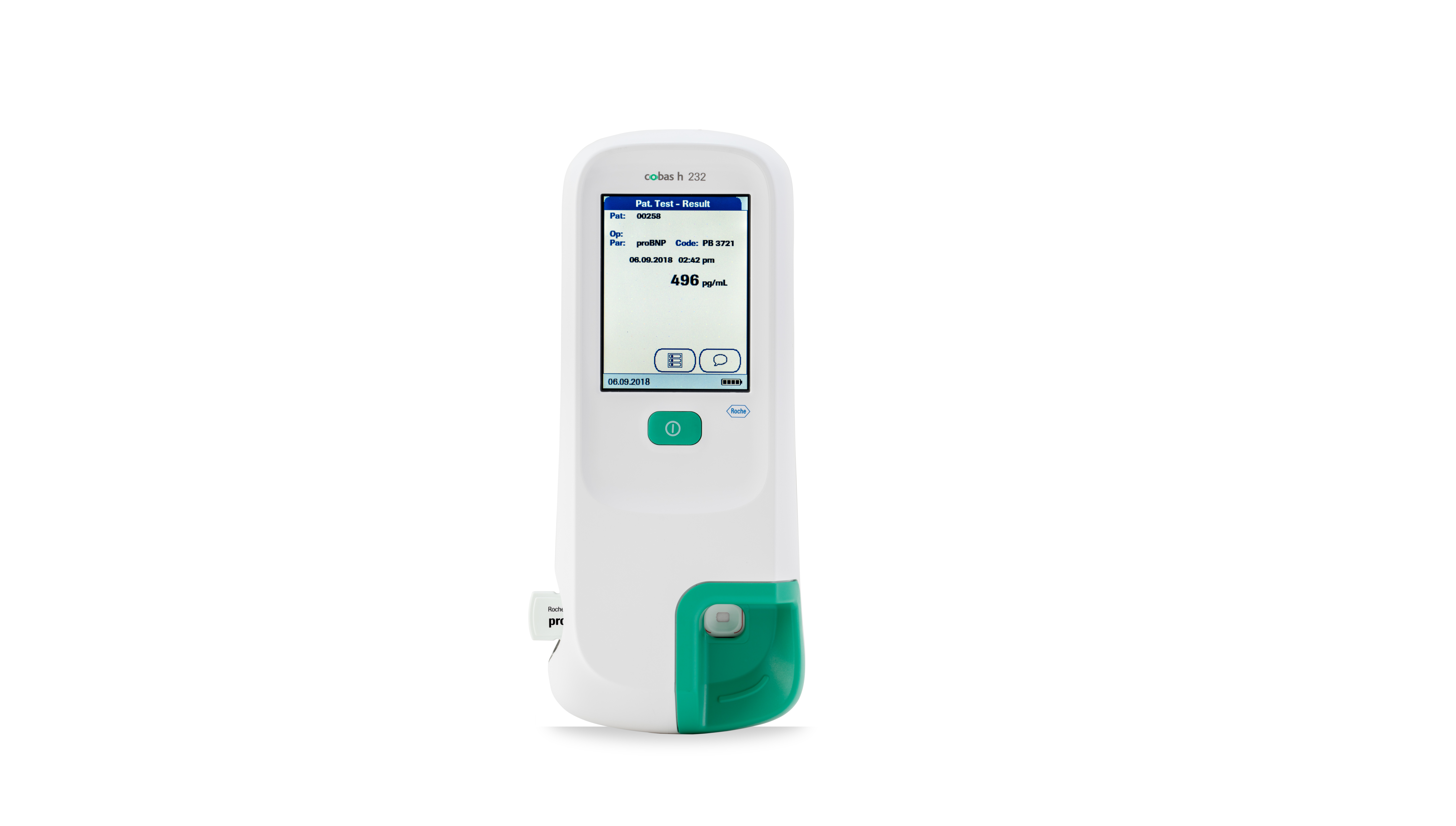
Consistent results across Point of Care and Laboratory settings
Our validated cobas® h 232 and Elecsys® NT-proBNP offering is standardized across the Point of Care (PoC) and the central laboratory, enabling consistent management wherever care is given, potentially without re-baselining patients' levels.44
Explore more
References:
- European Patent Office. EP 1 151 304 B1 patent document. Data on file.
- Roche. The NT-proBNP story. [Internet; cited 2024 July 17]. Available from: https://www.roche.com/stories/partnering-nt-probnp
- Fischer Y, et al. Evaluation of a New, Rapid Bedside Test for Quantitative Determination of B-Type Natriuretic Peptide. Clinical Chemistry. 2001;47:591-4.
- Roche Elecsys® NT-proBNP v2.1 method sheet. 2023-05, V 2.1.
- Roche CARDIAC proBNP+ method sheet. 2023-06, V 1.0.
- Abbott. Alere NT-proBNP method sheet. 2018-05, V1.0.
- Beckman Coulter. Access NT-proBNP method sheet. C99356 F. 2023-04, V 10.0.
- Siemens Healthineers. Atellica® NT‑proBNP method sheet. 2022-10, V 5.0.
- Biolabs Diagnostics. MagIumi NT-proBNP method sheet. V 3.0.
- Walkley R, et al. The cost-effectiveness of NT-proBNP for assessment of suspected acute heart failure in the emergency department. ESC Heart Fail. 2023;10:3276-3286.
- Walter E, et al. Cost-effectiveness of NT-proBNP-supported screening of chronic heart failure in patients with or without type 2 diabetes in Austria and Switzerland. Journal of Medical Economics. 2023;26:1287-300.
- Roche. Literature review. Search No. 24-0084. Data on file.
- Shahim B, et al. Global public health burden of heart failure: An Updated Review. Card Fail Rev. 2023;9:e11.
- Timmis A, et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur Heart J. 2020;41:12-85.
- Roth GA, et al. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736-88.
- Savarese G, et al. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2023;118:3272-87. Erratum: Cardiovasc Res. 2023 Jun 13;119(6):1453.
- Hasani WSR, et al. The global estimate of premature cardiovascular mortality: a systematic review and meta-analysis of age-standardized mortality rate. BMC Public Health. 2023;23:1561.
- Ponikowski P, et al. Heart failure: preventing disease and death worldwide. ESC Heart Fail. 2014;1:4-25.
- Virani SS, et al. Heart disease and stroke statistics – 2020 update: a report from the American Heart Association. Circulation. 2020;141:e139–e596.
- Lippi G, Sanchis-Gomar F. Global epidemiology and future trends of heart failure. AME Med J 2020;5:15.
- Roche Diagnostics. Cardiovascular disease and cardiac biomarkers. [Internet; cited 2024 July 17] Available from: https://diagnostics.roche.com/gb/en/products/product-category/cardiac-cardiac-markers.html
- Roche. Elecsys® ProBNP II method sheet. 2020-11, V 3.0.
- Mebazaa A, et al. Safety, tolerability and efficacy of up-titration of guideline-directed medical therapies for acute heart failure (STRONG-HF): a multinational, open-label, randomised, trial. Lancet. 2022;400:1938-52.
- Huelsmann M, et al. PONTIAC (NT-proBNP selected prevention of cardiac events in a population of diabetic patients without a history of cardiac disease): a prospective randomized controlled trial. J Am Coll Cardiol. 2013;62:1365-72.
- Writing Committee for the VISION Study Investigators, Devereaux PJ, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2017;317:1642-51.
- BioSpace. Roche Diagnostics Release: FDA Clears NT-proBNP Test For New Risk Stratification Use. [Internet; cited 2024 July 17]. Available at: https://www.biospace.com/article/releases/roche-diagnostics-release-fda-clears-nt-probnp-test-for-new-risk-stratification-use
- Januzzi JL, et al. NT-proBNP testing for diagnosis and short-term prognosis in acute destabilized heart failure: an international pooled analysis of 1256 patients: the International Collaborative of NT-proBNP Study. Eur Heart J. 2006;27:330-7.
- Januzzi JL, et al. N-Terminal Pro-B-Type Natriuretic Peptide in the emergency department: The ICON-RELOADED Study. J Am Coll Cardiol. 2018;71:1191-200.
- Singh H , et al. Types and Origins of Diagnostic Errors in Primary Care Settings. JAMA Intern Med. 2013;173:418-25.
- Hayhoe B, et al. Adherence to guidelines in management of symptoms suggestive of heart failure in primary care. Heart. 2019;105:678-85.
- Bottle A, et al. Routes to diagnosis of heart failure: observational study using linked data in England. Heart. 2018;104:600-5.
- Talha KM, et al. Use of natriuretic peptides and echocardiography for diagnosing heart failure. Eur J Heart Failure. 2024;26:551-560.
- Roche Diagnostics. Buzzback Heart failure in primary care report. 2022-08. Data on file.
- Roche Diagnostics. Censuswide The hidden costs of late diagnosis report. 2020-08. Data on file.
- Rawshani A, et al. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med. 2017;376:1407-18.
- Bayes-Genis A, et al. The 'Peptide for Life' Initiative: a call for action to provide equal access to the use of natriuretic peptides in the diagnosis of acute heart failure across Europe. Eur Heart J. 2021;23:1432-6.
- Bayes-Genis A, et al. The ‘peptide for life’ initiative in the emergency department study. ESC Heart Failure. 2024;11: 672-80.
- Roche. Partnering with Patients. [Internet; cited 2024 July 17]. Available from: https://www.roche.com/about/sustainability/patient-partnership#:~:text=Partnering%20with%20Patients&text=We%20define%20patient%20communities%20broadly,and%20family%20members%20of%20patients
- Fuery MA, et al. Prognostic impact of repeated NT-proBNP measurements in patients with heart failure with reduced ejection fraction. JACC Heart Failure. 2024;12:479-487.
- McDonagh T, et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2023;44:3627-39.
- Maddox T, et al. 2024 ACC Expert Consensus Decision Pathway for Treatment of Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2024;83:1444-88.
- Remme WJ, Swedberg K. Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J. 2001;22:1527-60.
- Heidenreich P, et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J Am Coll Cardiol. 2022;79:e263-421.
- Khan MS, et al. Trends in 30- and 90-day readmission rates for heart failure. Circ Heart Fail. 2021;14:e008335.
- Kimmoun A, et al. Temporal trends in mortality and readmission after acute heart failure: a systematic review and meta-regression in the past four decades. Eur J Heart Fail. 2021;23:420-31.
- Gheorghiade M, et al. Developing new treatments for heart failure: Focus on the heart. Circ Heart Fail. 2016;9:e002727.
- Greene SJ, et al. The vulnerable phase after hospitalisation for heart failure. Nat Rev Cardiol. 2015;12:220-9.