For localized information and support, would you like to switch to your country-specific website for {0}?
Giving you and your patients the answers you need—when and where you need them most
Infectious diseases are a global health threat with emerging pathogens and antimicrobial resistance presenting a growing challenge to personal and public health. Molecular Point of Care (POC) testing solutions are rapidly evolving as game changers in disease detection and infection control. They enable fast, accurate diagnoses right at the site of care for a range of common infectious diseases, allowing you to differentiate infections with similar symptoms.
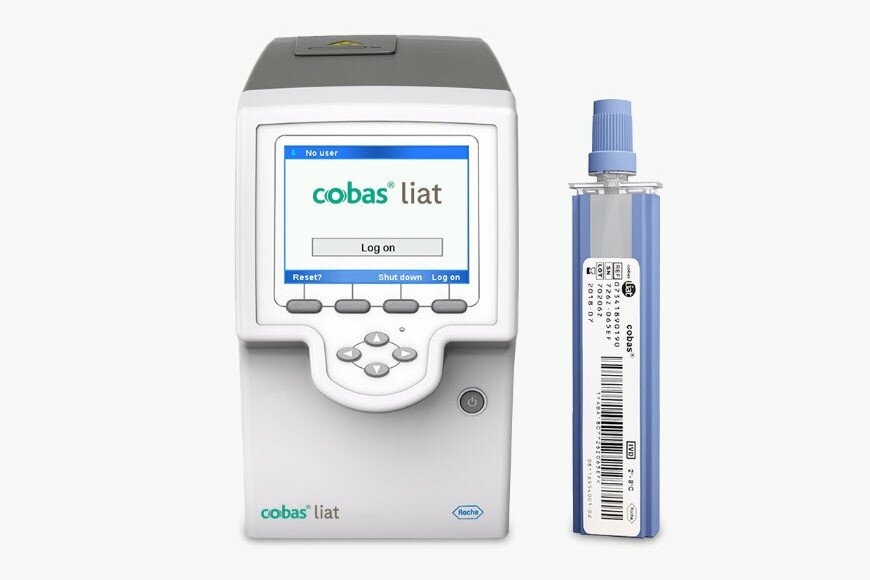
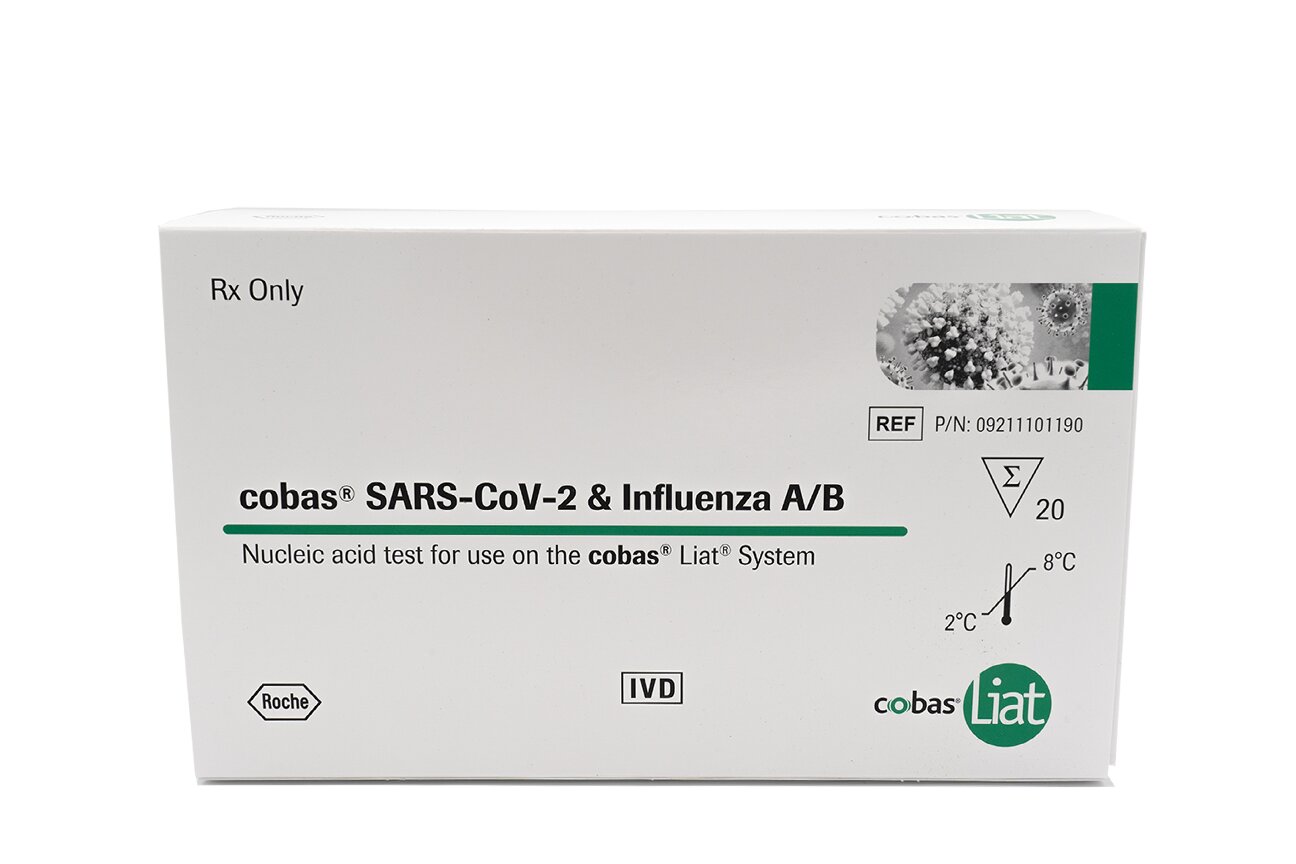
With the cobas® liat system you benefit from a quick and reliable PCR near-patient testing solution with frontline single and multiplex assays for the rapid diagnosis of COVID-19, influenza, respiratory syncytial virus (RSV), strep throat, and Clostridioides difficile (C. diff).
Together with our navify® POC digital solutions, we enable seamless access to fast results for targeted, quick testing and confident clinical decision-making. By equipping you with actionable insights right at the Point of Care, we empower you to make informed clinical decisions, enhance the patient experience, and help reduce disease transmission in hospitals and communities. All while providing centralized control to improve device and data management across different settings.
Featured products
Benefits of molecular Point of Care solutions from Roche
Enable fast, accurate test results to enhance healthcare decisions
Minimize additional testing: Rapid bedside PCR testing in the emergency department has been shown to significantly improve patient care. Physicians indicated that 64% of tests would change patient management, lowering the number of tests ordered by 18% and reducing emergency department (ED) length of stay by 33 minutes.1
Promote single appointment consultation: Evidence suggests molecular POC testing may facilitate faster and more accurate diagnosis, enabling targeted treatment during the initial patient visit. This approach has been shown to support improved outcomes when compared with central laboratory-based testing or syndromic management and empiric therapy.2,3
Use advanced POC technologies to help curb the spread of infections: During the COVID-19 pandemic, the World Health Organization released several RT-PCR protocols to ensure accurate diagnosis and aid in testing populations. These measures helped control the disease's spread.4
Help enable better patient outcomes with gold standard technology
Gold standard PCR technology: PCR testing for influenza in the emergency department is just as accurate as centralized lab tests, with both having about 98.8% sensitivity and 98.5% specificity.5
Support better patient outcomes: Medical evidence has shown that 99% of patients with influenza diagnoses supported by molecular point of care test results were given antiviral treatment within 5 days, compared to just 62% in the group without mPOC testing.6
Reduce waiting time and boost patient throughput compared to traditional lab diagnostics
Facilitate reduced emergency department (ED) waiting time and length of hospital stay: Evidence shows that the introduction of molecular POC testing can reduce in-hospital waiting time for results during isolated care and median length of stay.7
Drive improved patient flow: Studies reveal rapid molecular testing in EDs during COVID-19 significantly boosted patient throughput, with faster turnaround time and reduced ED occupancy rates.8
Provide centralized control: navify® POC digital solutions enable hospital staff to manage devices from a centralized lab or a decentralized location to orchestrate POC operations, enabling remote troubleshooting, simplifying software updates, and ongoing monitoring.
Support reduced costs and unnecessary medication use
Enable cost reduction by promoting earlier care intervention: Introducing POC testing for patients with viral respiratory symptoms can reduce diagnosis time, hospital stay, and in-hospital costs, studies suggest. A university hospital study tracked adult respiratory patients and found that a faster diagnostic tool could cut treatment time and save up to €293,471 in one influenza season.7
Help reduce unnecessary medication use: Studies have shown doctors changed treatment plans in 77.0% of cases after receiving a negative molecular POC influenza test, helping to avoid unnecessary antiviral prescriptions.9
Keeping operations running during unprecedented times
Related health topics
Images on this page are used for illustrative purposes only; any person depicted in the content is a model.
References
- Rogan DT et al. Impact of Rapid Molecular Respiratory Virus Testing on Real-Time Decision Making in a Pediatric Emergency Department. J Mol Diagn. 2017 May 19
- Burkins J et al. Factors associated with unsuccessful follow-up in patients undertreated for gonorrhea and chlamydia infections. Am J Emerg Med. 2020;38(4):715-719.
- Rolland C et al. Who fails to return within 30 days after being tested positive for HIV/STI in a free testing centre?. BMC Infect Dis. 2020;20(1):795.
- Fernandes RS et al. Recent advances in point of care testing for COVID-19 detection. Biomed Pharmacother. 2022;153:113538.
- Hansen GT et al. Clinical decision making in the emergency department setting using rapid PCR: Results of the CLADE study group. J Clin Virol. 2018;102:42-49.
- Clark TW et al. Clinical impact of a routine, molecular, point-of-care, test-and-treat strategy for influenza in adults admitted to hospital (FluPOC): a multicentre, open-label, randomised controlled trial. Lancet Respir Med. 2021;9(4):419-429.
- Rahamat-Langendoen J et al. Impact of molecular point-of-care testing on clinical management and in-hospital costs of patients suspected of influenza or RSV infection: a modeling study. J Med Virol. 2019;91(8):1408-1414.
- Baron A et al. Impact of Fast SARS-CoV-2 Molecular Point-Of-Care Testing on Patients' Length of Stay in an Emergency Department. Microbiol Spectr. 2022;10(4):e0063622.
- El Feghaly RE et al. Impact of Rapid Influenza Molecular Testing on Management in Pediatric Acute Care Settings. J Pediatr. 2021;228:271-277.e1.