By utilising a combination of rapid near patient and comprehensive high throughput laboratory based sexual health testing, you have the opportunity to create a tailored sexual health service to meet patient needs.
Roche sexual health solutions
Advanced STI testing solution
Create a tailored sexual health testing pathway
Right test, right place, right time
Sexually transmitted infection (STI) rates are rising rapidly – putting a strain on NHS services.1 Healthcare providers have opportunities to create STI pathways around patients – increasing access for at-risk groups.2
Roche Diagnostics is committed to developing advanced diagnostic solutions that address the challenges our sexual health services face, helping you to create a complete diagnostic pathway for sexually transmitted infections.
The comprehensive STI testing menu offered by the cobas® 5800/6800/8800 systems can be complemented by the rapid testing provided by cobas® liat STI assays to provide a tailored and targeted STI testing pathway for both laboratories and clinics.
Comprehensive laboratory-based and rapid point of care testing

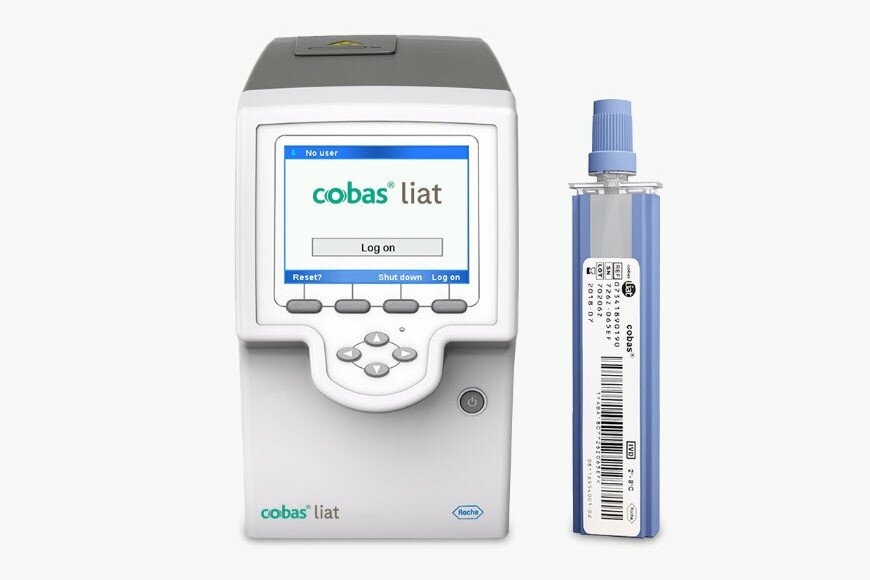
Point of Care Testing

cobas® liat system
Targeted, near patient testing
Quick results for one or several suspected pathogens
- Rapid results for treatment within a single appointment window
Laboratory Solutions

cobas® 5800/6800/8800
Comprehensive sexual health testing menu
Routine efficiency for symptomatic and asymptomatic screening
Resistance testing to address the increasing threat of antimicrobial resistance (AMR)*
Both the cobas® liat system and cobas® 5800/6800/8800 assays offer a gold standard PCR result, regardless of the patient journey. Standardised collection device for both urine and vaginal swabs
* NG resistance and MG resistance in development. For research use only.
References
1. "Sexually Transmitted Infections and Screening for Chlamydia in England: 2023 Report.” GOV.UK,
2. “Recommendations: Reducing Sexually Transmitted Infections: Guidance.” NICE, Accessed 21 Jan. 2025. www.nice.org.uk/guidance/ng221/chapter/Recommendations#improvinguptake-and-increasing-the-frequency-of-sti-testing.
3. “Recommendations: Reducing Sexually Transmitted Infections: Guidance.” NICE, . Accessed 21 Jan. 2025. www.nice.org.uk/guidance/ng221/chapter/Recommendations#improvinguptake-and-increasing-the-frequency-of-sti-testing
4. Jensen, Jorgen S., and Magnus Unemo. “Antimicrobial Treatment and Resistance in Sexually Transmitted Bacterial Infections.” Nature News, Nature Publishing Group, 20 Mar. 2024, www.nature.com/articles/s41579-024-01023-3. Accessed 21 Jan. 2025.
5. cobas® liat CT/NG/MG package insert 10147563190-01EN Doc Rev. 1.0 October 2024
6. cobas® liat CT/NG package insert 10147580190-01EN Doc Rev. 1.0 October 2024
7. UK Health Security Agency. STI prioritisation framework. Available at: Accessed: March 2025. https://assets.publishing.service.gov.uk/media/67376f07abe1d74ea7dade2d/STIprioritisation-framework.pdf
8. Huppert JS, et al. Improving notification of sexually transmitted infections: a quality improvement project and planned experiment. Pediatrics. 2012;130(2):e415-22.
9. Widdice LE, Hsieh YH, Silver B, Barnes M, Barnes P, Gaydos CA. Performance of the Atlas Genetics Rapid Test for Chlamydia trachomatis and Women's Attitudes Toward Point-Of-Care Testing. Sex Transm Dis. 2018 Nov;45(11):723-727.
10. Jensen, Jorgen S., and Magnus Unemo. “Antimicrobial Treatment and Resistance in Sexually Transmitted Bacterial Infections.” Nature News, Nature Publishing Group, 20 Mar. 2024
11. British Association for Sexual Health and HIV (BASHH). Standards for STI management. Available at: https://www.bashh.org/_userfiles/pages/files/bashhstandardsforstimanagement2019.pdf Accessed: Jan 2025.