For localized information and support, would you like to switch to your country-specific website for {0}?
Hospital del Mar, one of the first hospitals in Spain to adopt digital pathology, has relied on Roche Tissue Diagnostics instruments for more than a decade.
In 2013, this leading hospital implemented its first digital pathology system with VENTANA iScan HT slide scanner*, VENTANA Virtuoso* and the VENTANA Companion Algorithm image analysis software*. Since then, Hospital del Mar has made great strides with digital pathology and has been instrumental in shaping the digital pathology market in Spain. During the COVID-19 global pandemic, the pathology team has gone fully digital for case review. This busy laboratory team of 15 pathologists, 10 biologists and three hematologists review and diagnose about 30,000 biopsies and 25,000 cytologies annually, with 150,000 tissue slides evaluated each year.
We recently chatted with Dr. Belen Lloveras, Chief of the Pathology Department, on the impact of Roche Digital Pathology in cancer diagnosis during the global COVID-19 pandemic and beyond.
At Hospital del Mar

15 pathologists on staff

30,000 biopsies annually

25,000 cytologies annually

150,000 tissue slides annually
Prior to the pandemic, what did digital pathology look like at Hospital del Mar?
We first implemented digital pathology in 2013 with the VENTANA iScan HT slide scanner, starting with one pathologist diagnosing breast pathology on scanned slides. Over the next three years, we added pulmonary pathology, uropathology and some gastrointestinal samples, incorporating about 40 percent of our cases. We started scanning gynecologic pathology and dermatopathology, and just before the pandemic hit, we were digitizing more than 50 percent of our cases, with most pathologists having access to scanned slides.
How has COVID-19 impacted your utilisation of digital pathology?
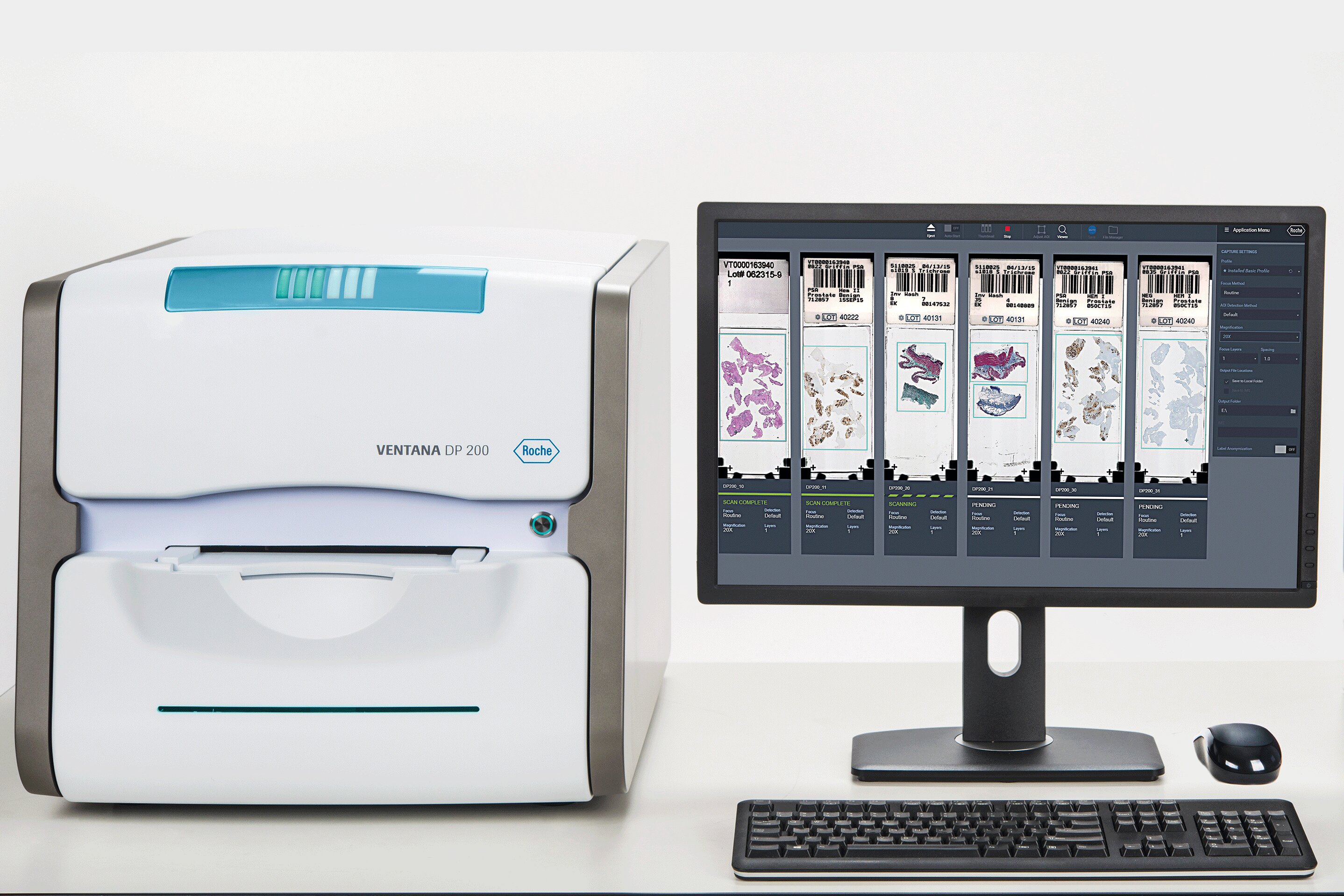
During the early months of the pandemic, few surgical operations and endoscopies were performed in the hospital, which resulted in fewer samples to be processed and scanned. This provided a great opportunity to scan all types of samples, to train most technicians and to acquire expertise in different types of samples and skills using iScan HT. Virtually all of our pathologists are able to use digital pathology routinely in diagnosis. We are now planning for 100 percent of cases to be scanned and incorporating VENTANA DP 200 slide scanners** into our workflow with Roche uPath enterprise software.
What has been the experience with your transition to using the VENTANA DP 200 slide scanner?
Before making the transition, we worked with Roche Diagnostics Spain to confirm that our DP 200 images reach the quality we were used to working with. We started with pulmonary pathology and research slides and our IT team secured the informatic connections with the Laboratory Information System (LIS) and with VENTANA Virtuoso image management software. When the number of slides increased again, we selected cases for the DP 200 based on their urgent nature as well as the quantity of slides, and the rest were still being scanned with VENTANA iScan HT.

Digital Pathology at Hospital del Mar
Dra. Belen Lloveras (seated) and Dra. Mar Iglesias pose with their iScan HT, in use at Hospital del Mar since 2013.
How has the Roche solution helped your team to conduct remote diagnosis during the COVID-19 pandemic? Can you share with us stories or anecdotes of success?
Due to social distancing and health concerns, the pandemic led many pathologists to set aside their microscope. Many professionals had one at home but slides are not allowed to travel outside the hospital on a regular basis. With digital pathology, pathologists became comfortable making diagnoses from home.
The surprise for some clinicians and surgeons came in the simple sharing of digital scans remotely during tumor boards. Digital pathology was also helpful in connecting with colleagues in our own department and to teach our residents from home using remote access through video conferences.
There is one anecdote I would like to share - as you already know, digital pathology allows pathologists to easily show images through NAVIFY Tumor Board. We have been taking pictures from digitized cases to be presented in tumor boards for many years and clinicians were accustomed to seeing the images. One day, we didn’t have time to create the images for a special case and the senior oncologist involved asked us why we didn’t show images. He missed them! It’s fantastic to teach our clinicians and digital pathology allows it and makes collaboration easier.

The Shift to Digital Pathology
During the COVID-19 global pandemic, the anatomic pathology team has gone fully digital for case review. Here, Dra. Mar Iglesias, reviews slides using uPath enterprise software.
How have Roche products helped you and your team overcome barriers to adoption of digital pathology?
Roche has been instrumental in implementing digital pathology in our Pathology Department. Their support in the hardware set-up and maintenance is important as hospital engineers typically are not trained in this type of equipment. Regarding integration with the department LIS, the Roche team has been leading and coordinating the entire process. These are challenges that can vary from hospital to hospital and close interaction between teams is key.
Explore more
* Ventana test systems are for in vitro diagnostic use for specific clinical applications, and are intended for research use only for other applications.
** The VENTANA DP 200 slide scanner is CE-IVD marked - Research Use Only in the USA.
VENTANA and NAVIFY are trademarks of Roche.