For localized information and support, would you like to switch to your country-specific website for {0}?
cobas® Cdiff

See C. diff sooner, start patient care faster
The cobas® Cdiff is a real-time PCR test that provides accurate, on-demand results in 20 minutes for the detection of toxigenic C. diff in unformed stool specimens from patients suspected of having a C. diff infection (CDI).
cobas® Cdiff
- Empowering faster informed isolation precautions with results in just 20 minutes
- Reducing the risk of missing an acute infection with enhanced toxigenic C. diff strain coverage
- Expanding C. diff testing beyond the lab with complete cobas® liat system connectivity
The cobas® liat system enables definitive and rapid diagnosis, combining powerful PCR technology with fast turnaround time, 20 minutes or less, allowing sample to answer while patients wait.
cobas® Cdiff performance
View full tablecobas® Cdiff performance
| Sensitivity* | Specificity* | |
|---|---|---|
| Cdiff LOD: 45-90 CFU/swab |
93.5 % (95% CI: 88.1% - 96.5%) |
96.4 % (95% CI: 94.6% - 97.7%) |
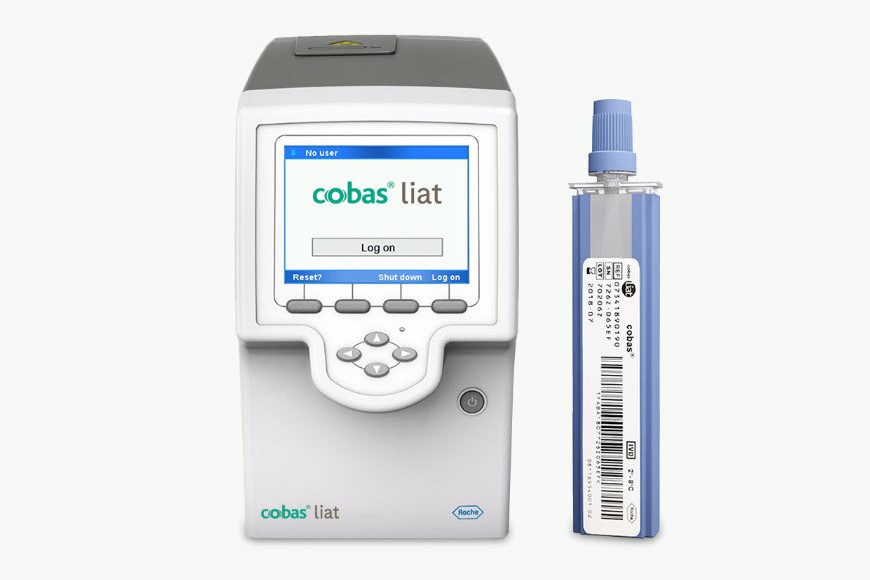
cobas® liat system
Rapidly test, triage, and treat your patients with the accuracy, simplicity, and security needed at the point of care.
Learn moreTraditional testing methods vs. cobas® liat system
Traditional diagnostic methods are either time-consuming, labor-intensive or inaccurate when used alone. Testing algorithms have been developed to overcome the shortcomings of individual methods. However, these algorithms are complex and can introduce delays in result reporting, which may adversely impact patient management.
Traditional testing
methods

Patient with CDI symptoms

Isolate

Collect sample and send to lab

Process with lab PCR or algo EIA

Positive results: initiate antibiotics
Negative results: admit patient without
isolation and search for symptom etiologies
cobas® Cdiff
test

Patient with CDI symptoms

Collect sample and send to lab

The cobas® liat system provides
accurate results in only 20 minutes

Positive results: isolate and initiate antibiotics
Negative results:, admit patient without
isolation and search for symptom etiologies
See C. diff sooner with cobas® Cdiff to transform your patient care pathway.
Relying on the fast, accurate results from cobas® Cdiff, healthcare professionals are now able to streamline CDI diagnosis to reduce unnecessary isolation, empiric use of antibiotics and to initiate appropriate infection control measures.
Intended Use
The cobas® Cdiff Nucleic acid test for use on the cobas® Liat® System is an automated, qualitative in vitro diagnostic test that uses real-time polymerase chain reaction (PCR) for the detection of the toxin B (tcdB) gene of toxigenic Clostridioides difficile in unformed (liquid or soft) stool specimens obtained from patients suspected of having C. difficile infection (CDI). The cobas® Cdiff Nucleic acid test for use on the cobas® Liat® System is intended for use as an aid in the diagnosis of CDI in humans in conjunction with clinical and epidemiological risk factors.
The cobas® liat system is commercially available in select markets. This product is not registered as an in vitro diagnostic (IVD) in all countries; additional information may be available from your Roche sales representative.
Registration status
CE-IVD, FDA 510(K) cleared
The cobas® liat system is commercially available in select markets. This product is not registered as an in vitro diagnostic (IVD) in all countries; additional information may be available from your Roche sales representative.
Package inserts
Access package inserts through your country’s Roche Diagnostics Website.
References
- https://cdifffoundation.org/2014/11/24/c-difficile-infection-cdi-european-clinician-consensus-report-with-recommendations-for-improved-management-of-cdi, accessed on Nov, 2023.
- Gupta A, et. al. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clin Proc. 2014 Nov;89(11):1525-36. doi: 10.1016/j.mayocp.2014.07.012. Epub 2014 Sep 20. PMID: 25245597.
- cobas® Cdiff [package insert v4], Pleasanton, CA; Roche Molecular Systems, Inc., 2022
COBAS and LIAT are trademarks of Roche.
?wid=1920&hei=768&fmt=png-alpha&fit=crop,1)

