For localized information and support, would you like to switch to your country-specific website for {0}?
Driving diagnostic certainty for life-changing decisions in breast cancer management
Committed to delivering quality diagnostics without compromise
For decades Roche has been at the forefront of advancing breast cancer diagnostics, dedicated to enhancing patient care with precision and reliability. Each breast tissue sample represents an individual’s journey, and we are committed to providing healthcare providers with the most accurate and advanced tools to support their crucial work in diagnosing and managing breast cancer.
Roche’s comprehensive diagnostics portfolio offers a range of solutions, including immunoassays, molecular tests, tissue diagnostics, and digital solutions. These tools work together to provide a thorough understanding of breast cancer, from initial detection to ongoing management, empowering healthcare providers to make informed decisions with confidence.
Partnering with Roche means access to a wealth of experience, cutting-edge technology, and a relentless commitment to improving patient outcomes. We continuously invest in and refine our diagnostics to enhance sensitivity and accuracy, ensuring that healthcare providers are equipped with the best possible tools.
The most common cancer among women worldwide1
Female breast cancer has surpassed lung cancer as the most commonly diagnosed cancer worldwide, claiming more than 600,000 lives each year.1,2 The incidence of breast cancer is rising and by 2040 the incidence of new breast cancers is predicted to be more than three million per year, rising most rapidly in low- and middle-income countries (LMICs).3
The economic cost of breast cancer is estimated to be $2 trillion between 2020 and 2050 making it the third most expensive cancer globally.4 There are also the so-called “hidden costs,” including the financial, physical, psychological, emotional, and social impact that breast cancer has on children, families, communities, and the greater society as a whole.3
Advances in early detection, innovative testing, and new therapies are helping to strike a powerful blow against this disease. From 1989 to 2016, the number of breast cancer-related deaths dropped by 42%, but there is still much work to be done.5
Barriers to diagnosis create disparities in care
Prognosis for breast cancer patients becomes worse as it advances, therefore it is crucial to diagnose and start appropriate treatment as early as possible. Unfortunately, there are disparities in diagnosis globally. While late-stage diagnosis occurs in around 6% of women in the United States, around 75% of sub-Saharan African women are diagnosed at a late stage when treatment is less effective.6 Barriers to diagnosis include timeliness, access, and accuracy:
- Timely diagnosis: Individual factors such as poor cancer awareness and sociocultural taboos, disease-related factors such as the clinical manifestation and growth of tumors, as well as health system constraints can be challenging for the timely diagnosis of breast cancer, particularly in low- and middle-income countries.7,8
- Access to diagnostics: the complexity of cancer means that access to high-quality diagnostics can be challenging even in high-income countries, which in turn may result in limited treatment options.7,8
- Accuracy of diagnosis: an additional challenge to diagnosis is the quality of initial diagnostic and staging procedures. For an accurate diagnosis, steps such as adequate biopsy procedures, proper handling of the obtained material, and availability of basic pathology and immunohistochemistry are essential, but are not always available.7
We are working closely with governments and policymakers to address these challenges. It is more important than ever to work together to improve access to timely, accurate screening and diagnosis for people all over the world, especially in resource-limited regions.9
Featured products
Benefits of Roche diagnostic solutions for managing breast cancer
A trusted foundation for delivering innovation
Roche is working to develop diagnostic solutions that will bring us closer to fulfilling our long-term ambition of offering patients everywhere precise, personalized treatment approaches.
Trusted expertise: With 120 years of experience in driving innovation, our strong foundation enables us to offer a comprehensive, sensitive, and clinically actionable breast cancer diagnostics portfolio to consistently deliver the confident results patients need. As an example, our HER2 (4B5) antibody, launched in 2006, has shown the most consistent performance and superior quality when compared to other on-market HER2 clones, empowering healthcare professionals to provide reliable results.10
Evolving innovation: As our understanding of breast cancer grows, our portfolio of sensitive and clinically actionable solutions evolves with it.
- Roche HER2 (4B5) assay: Building on proven technology this widely adopted assay has gained approval to identify HER2-low breast cancer patients, who account for 45–55% of breast cancer patients, meaning even more women can be matched with highly effective personalized therapies.11,12
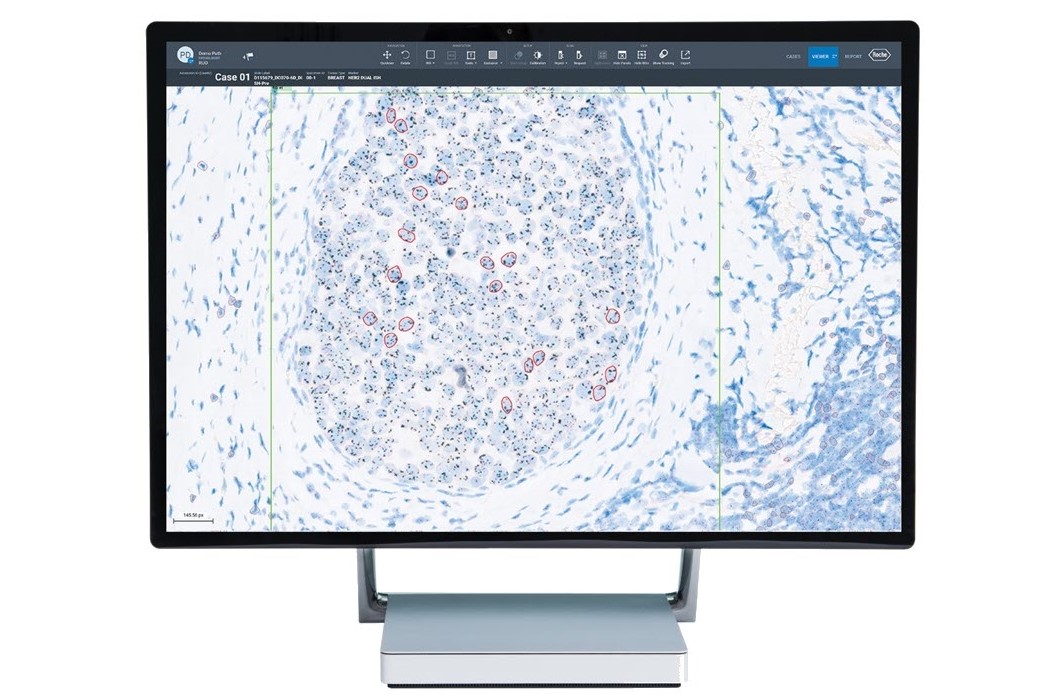
- Pioneers in digital pathology: Roche was the first company to offer a comprehensive portfolio of FDA-cleared image analysis algorithms and digital read applications for the five key immunohistochemistry (IHC) breast markers.13
- In 2020, IDEA Pharma rated Roche #1 for innovation, citing several exciting milestones for our PD-L1 (SP142) assay, including a groundbreaking test and treatment for triple-negative breast cancer (TNBC).14
Investing in the future: In 2022 Roche invested the most in research and development among the top in-vitro diagnostic companies to ensure we continue to innovate in diagnostics.15
Empowering healthcare providers with comprehensive diagnostic solutions
Better diagnostic tools lead to greater clinician confidence and improved patient care. That is why our comprehensive breast cancer solutions portfolio provides all the diagnostic assays and companion diagnostic biomarkers needed throughout the patient journey.
- Diagnosis and typing: Tissue-based biomarkers play an integral role in aiding pathologists in diagnosing and typing breast cancer cases. Roche Diagnostics provides several primary antibodies to aid in the diagnosis and typing of breast cancer, like the VENTANA anti-Ecadherin (36) Mouse Monoclonal Primary antibody which may be used to aid in the differentiation of in situ and/or invasive lobular carcinoma from in situ and/or invasive ductal carcinoma of the breast.16
- Prognosis: Biomarkers that can aid in the management, prognosis, and prediction of hormone therapy for breast carcinoma, such as CONFIRM anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody and CONFIRM anti-Progesterone Receptor (PR) (1E2) Rabbit Monoclonal Primary Antibody.17,18
- Treatment decision: For nearly 25 years, Roche has been at the forefront of innovating companion diagnostics (CDx) that provide essential information to inform clinical decision-making and guide personalized treatment strategies.19 These include our legacy HER2 (4B5) antibody, the VENTANA HER2 Dual ISH DNA Probe Cocktail that is indicated as an aid in the assessment of patients for whom Herceptin (trastuzumab) is being considered, and the VENTANA PD-L1 (SP142) Assay, the first and only companion test to identify patients with triple-negative breast cancer (TNBC) for immunotherapy treatment with TECENTRIQ (in countries accepting the CE Mark only).20,21,22
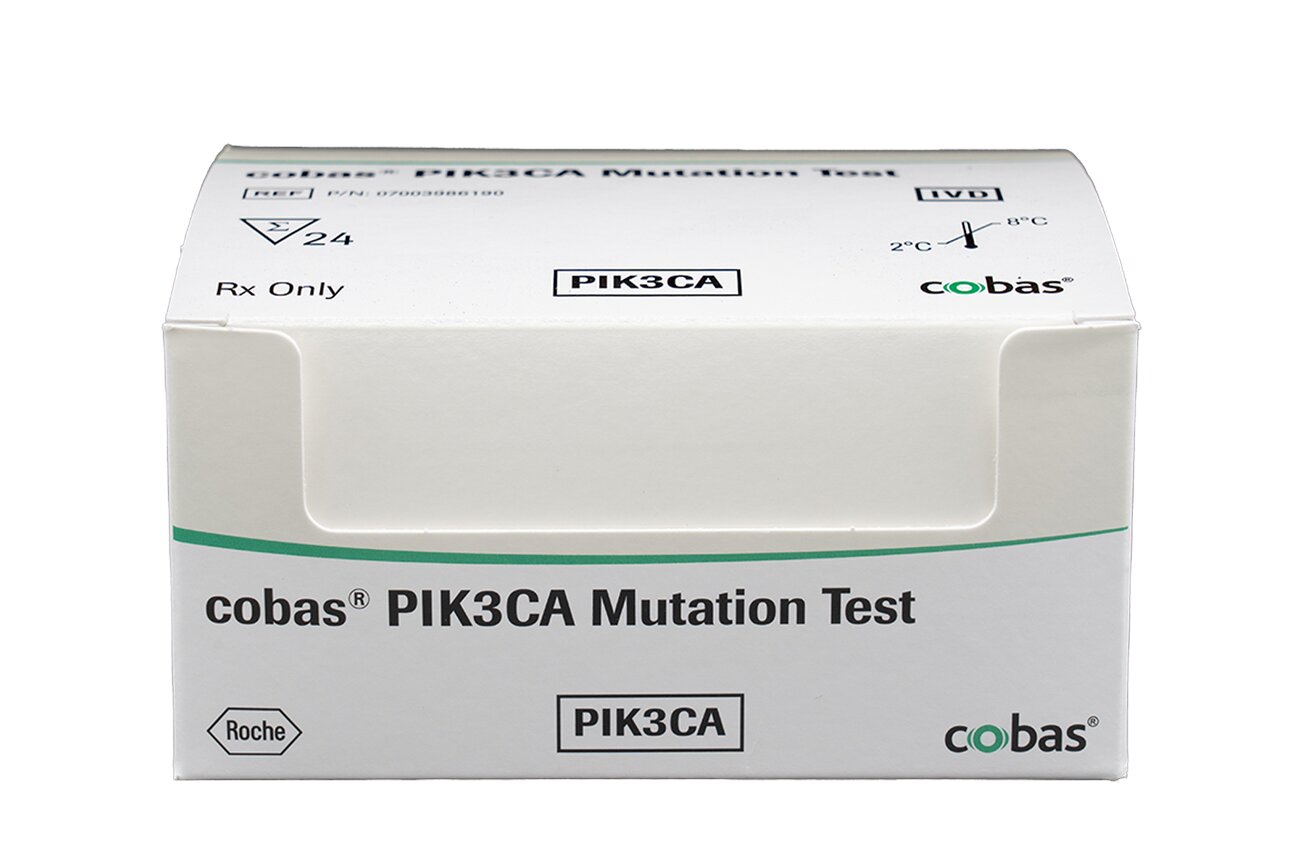
Additionally, as new targeted treatments become available, the detection of specific mutations using molecular methods is becoming more important. Recently, the FDA granted Roche a breakthrough therapy designation for the breast cancer therapy Itovebi™ (inavolisib), based on positive results from its recent Phase III trial for the treatment of adult patients with PIK3CA-mutated, hormone receptor-positive, HER2-negative breast cancer.23 The cobas® PIK3CA Mutation Test detects 17 mutations in exons 2, 5, 8, 10, and 21 in the PIK3CA gene and offers high-quality, fast results, improving laboratory efficiency and allowing healthcare professionals to make confident treatment decisions sooner.24 Just recently, Foundation Medicine, Inc. announced that it has received approval from the FDA for FoundationOne®Liquid CDx to be used as a companion diagnostic for Itovebi™ (inavolisib).25
- Early detection of recurrence and monitoring response to treatment, such as the Immunoassay Elecsys® CA 15-3 II: Immunological in vitro assay for the quantitative determination of CA 15 3 in human serum, Li heparin and EDTA plasma to aid in the management of breast cancer patients. In conjunction with other clinical and diagnostic procedures, serial testing with this assay is an aid in the early detection of recurrence in previously treated stage II and III breast cancer patients, and for monitoring response to therapy in metastatic breast cancer patients.26
- Clinical decision: Roche Diagnostics is committed to helping healthcare providers in clinical decision-making for better patient care. Image analysis algorithms have the potential to enhance the concordance of challenging cases in breast cancer. As an example, the uPath HER2 Dual ISH image analysis is intended for use as an aid to the pathologist to determine HER2 gene status and, when used with the VENTANA HER2 Dual ISH Assay, it is indicated as an aid in the assessment of breast cancer patients for whom Herceptin®(trastuzumab) treatment is being considered.13
Collaborations and partnerships to help create better outcomes for all
Ongoing partnerships:
- We have more than 85 ongoing collaborations with 95+ pharma partners to develop companion cancer diagnostics that advance personalized treatment.19
- Additionally, our collaborations with vendors on digital breast cancer solutions play an increasing role such as the Roche Digital Pathology Open Environment which brings together a wide array of innovative AI-based pathology tools to aid clinicians. Breast cancer collaborators include:27
- DiaDeep: Algorithms for breast cancer biomarker quantification
- Mindpeak: Algorithms for breast biomarkers and pan tumor PD-L1 for lung, gastric, esophageal, bladder, and breast cancers
- Stratipath: Algorithm for risk profiling of invasive breast cancer
Expanding education and access:28
- Rapid detection and treatment of breast cancer, particularly at an early stage is critical for maximizing the chances of a positive outcome but we recognize access to healthcare services varies widely.
- Supporting grassroots programs is an effective route to long-term change. We have seen this with our EMPOWER program in Kenya which has brought together public, private, and not-for-profit organizations to improve education around cancer and expand access to breast and cervical cancer screening and treatment.
- To help improve access in urban areas, we have partnered with City Cancer Challenge (C/Can), a not-for-profit organization working with cities around the world to improve access to quality, equitable care.
- Another geographical-based initiative we are proudly partnering on is the Johns Hopkins Program for International Education in Gynecology and Obstetrics (Jhpiego) in support of the World Health Organization’s Global Breast Cancer Initiative to save 2.5 million lives from breast cancer by 2040. As part of this effort, Roche aims to increase access to cervical cancer and breast cancer diagnostics and treatment in sub-Saharan Africa, by collaboratively providing technical and clinical guidance to local healthcare providers, while working with local decision makers to support policy adoption and financing for education, screening, and patient care programs.
Collaborating to close the gaps in women’s health:
- Roche’s XProject is a long-term commitment and an ongoing initiative to drive meaningful change through partnerships, funding and action to help close the gaps in women’s health for better health outcomes for everyone. Our initiatives include:29
- Shaping ecosystems: A public partnership to improve health and well-being of women in Egypt, focusing on early detection and treatment of breast cancer
- Partnering for innovation: Partnering with different FemTech organizations to advance innovative solutions in women’s health
- Offering women-centric approaches: Developing Affordability and Capacity partnerships in Ivory Coast, to overcome system challenges and provide integrated care for breast & cervical cancer
Explore more
References
- World Health Organization. International Agency for Cancer Research. Cancer Today: Data Sheet [Internet; cited 2024 Oct 24] Available from: https://gco.iarc.fr/today/en/dataviz/tables?mode=cancer&group_populations=1&multiple_populations=1&sexes=2
- World Health Organization. International Agency for Cancer Research. Cancer Today: Data Sheet [Internet; cited 2024 Oct 24] Available from: https://gco.iarc.fr/today/en/dataviz/tables?mode=cancer&group_populations=1&multiple_populations=1&sexes=2&types=1
- Coles CE, et al. The Lancet Breast Cancer Commission. The Lancet. 2024 May 11; 403 (10439):1895-1950
- Chen S, et al. Estimates and Projections of the Global Economic Cost of 29 Cancers in 204 Countries and Territories From 2020 to 2050. JAMA Oncol. 2023 Feb 23;9(4):465-472.
- American Cancer Society. About Breast Cancer. [Internet; cited 2024 Oct 24] Available from:https://www.cancer.org/cancer/types/breast-cancer/about/how-common-is-breast-cancer.html
- Elima Jedy-Agba et al, Stage at diagnosis of breast cancer in sub-Saharan Africa: a systematic review and meta-analysis. The Lancet. 2016; 12: E923-E935
- Nnaji CA, et al. Timeliness of diagnosis of breast and cervical cancers and associated factors in low-income and middle-income countries: a scoping review. BMJ Open. 2022;12:e057685.
- Dos-Santos-Silva I, et al. Global disparities in access to cancer care. Communications Medicine. 2022 Apr 07; 2:31
- F. Hoffmann-La Roche Ltd. Annual Report 2023 [Internet; cited 2024 Oct 24]. Available from: https://www.roche.com/investors/annualreport23
- Roche Diagnostics Ltd. Data on file. MC-12674
- Tarantino P, et al. HER2-Low Breast Cancer: Pathological and Clinical Landscape. JCO. 2020 Apr 24; 38(17): 1951-1962
- Garrido C, et al. Analytical and clinical validation of PATHWAY Anti HER 2/neu (4B5) antibody to assess HER2 low status for trastuzumab deruxtecan treatment in breast cancer. Virchows Archiv. 2024 Oct 20; 484:1005-1014.
- PR Newswire. Ventana receives FDA clearance for Estrogen Receptor (ER) Image Analysis and Digital Read Application for breast cancer [Press release; cited 2024 Nov 26]. Available from: https://www.prnewswire.com/news-releases/ventana-receives-fda-clearance-for-estrogen-receptor-er-image-analysis-and-digital-read-application-for-breast-cancer-234579611.html
- Idea Pharma Pharmaceutical Innovation and Invention Index [Internet; cited 2024 Nov 26]. Available from: https://www.ideapharma.com/pii/#roche
- F. Hoffmann-La Roche Ltd. Data on file.
- F. Hoffmann-La Roche Ltd. VENTANA® anti-E-cadherin (36) Mouse Monoclonal Primary Antibody Method Sheet. 2023.
- F. Hoffmann-La Roche Ltd. CONFIRM anti-Estrogen Receptor (ER) (SP1) Rabbit Monoclonal Primary Antibody Method Sheet. 2023.
- F. Hoffmann-La Roche Ltd. CONFIRM anti-Progesterone Receptor (PR) (1E2) Rabbit Monoclonal Primary Antibody Method Sheet. 2023.
- Roche Diagnostics. Data on file. MC--12507
- F. Hoffmann-La Roche Ltd. VENTANA® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody Method Sheet. 2023.
- F. Hoffmann-La Roche Ltd. VENTANA® HER2 Dual ISH DNA Probe Cocktail Method Sheet. 2023
- F. Hoffmann-La Roche Ltd. VENTANA® PD-L1 (SP142) Assay Method Sheet. 2023.
- Pharmaceutical Technology. Roche’s PI3K inhibitor secures breakthrough status in breast cancer [News article; cited 2024 Oct 24]. Available from: https://www.pharmaceutical-technology.com/news/roches-pi3k-inhibitor-secures-breakthrough-status-in-breast-cancer/
- F. Hoffmann-La Roche Ltd. cobas® PIK3CA Mutation Test Method Sheet. 2024
- FoundationOne® Liquid CDx [Technical Information; cited 2024 Nov 24] Available from: https://info.foundationmedicine.com/hubfs/FMI%20Labels/FoundationOne_Liquid_CDx_Label_Technical_Info.pdf
- F. Hoffmann-La Roche Ltd. (2024) Elecsys® CA 15-3 II Method Sheet
- F. Hoffmann-La Roche Ltd. Roche advances AI-driven cancer diagnostics by expanding its digital pathology open environment [Internet; cited 2024 Oct 24]. Available from: https://diagnostics.roche.com/global/en/news-listing/2024/roche-advances-ai-driven-cancer-diagnostics-by-expanding-its-digital-pathology-open-environment.html
- F. Hoffmann-La Roche Ltd. Breaking down barriers to health equity in breast cancer [Internet; cited 2024 Oct 24]. Available from: https://www.roche.com/stories/breast-cancer-health-inequity
- F. Hoffmann-La Roche Ltd. XProject & Women’s Health FAQ. [Internet; cited 2024 Oct 24]. Available from: https://www.roche.com/xproject/faq#4c5c55b7-9f3b-4a3e-b9e8-9d4194cad0ff