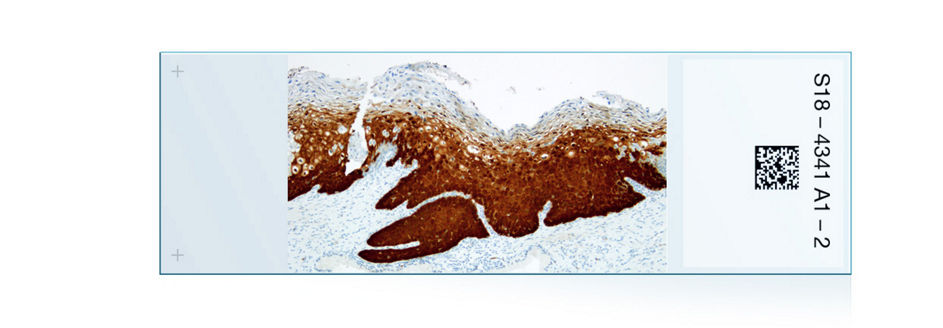
The (CERvical Tissue AdjunctIve aNalysis) study is one of the largest, most rigorous immunohistochemistry clinical studies.
As a result of the CERTAIN Study, CINtec® Histology achieved ≥ 99% acceptability for staining, morphology, and background.1 CAP (College of American Pathologists), the ASCCP and WHO (World Health Organization) recommend the adjunctive use of p16 IHC in evaluation of cervical biopsies.
Download the CINtec Histology CERTAIN study brochure.