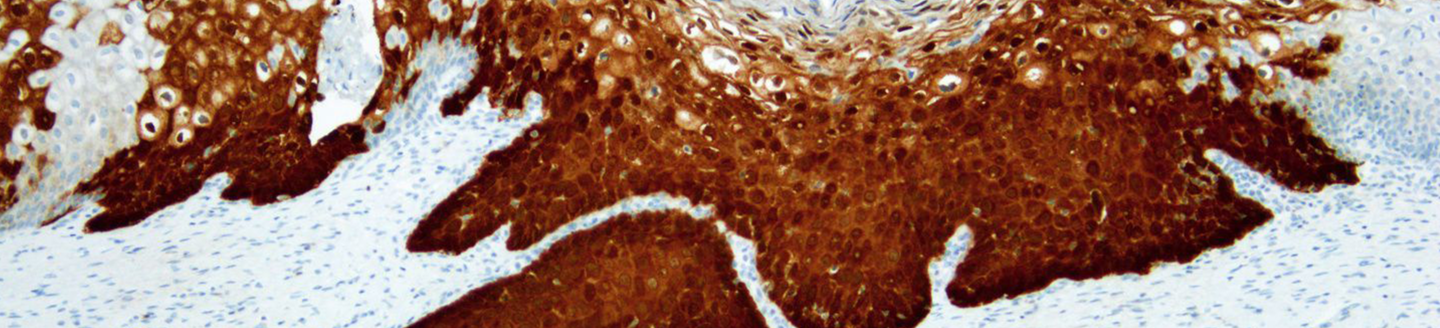
CINtec® Histology

CINtec® Histology: be conclusive in diagnosing cervical precancer
CINtec® Histology provides objectivity to diagnostic interpretation that helps all pathologists identify more cervical disease. The CINtec® Histology test is the only p16 biomarker test CE marked and U.S. 510(k) cleared for clinical use in the evaluation of cervical biopsy specimens. Pathologists who use CINtec® Histology demonstrate improved diagnostic consistency and diagnostic agreement between each other and with expert gynecopathologists.1
CINtec® Histology enhances identification of occult lesions that may be missed by H&E or morphologic interpretation alone, adding objectivity to cervical biopsy interpretation to help pathologists make informed diagnoses. The adjunctive use of CINtec® Histology helps pathologists ensure the right patient is treated without unnecessarily treating more patients.

When CINtec® Histology is used according to the LAST recommendations*:

Diagnostic sensitivity improves by 11.8% and specificity improves by 9.7% to identify high-grade cervical disease1

Diagnostic consistency for high-grade disease of challenging cases by the majority of pathologists improves by 29.5%1

Pathologists who use CINtec® Histology demonstrate improved diagnostic consistency with each other and with expert gynecopathologists.1
Ordering information
CINtec® Histology - U.S. 510(k) |
||||||
| System Compatiblity | ||||||
Product Name** |
Material Number |
Number of Tests |
BenchMark ULTRA |
BenchMark XT | BenchMark GX | Manual/Semi- automated Processors |
CINtec® Histology |
06680003001 | 50 | ✔ | - | - | - |
CINtec® Histology |
06680011001 | 250 | ✔ | - | - | - |
CINtec® p16 Histology - CE/IVD |
||||||
| System Compatiblity | ||||||
Product Name** |
Material Number |
Number of Tests |
BenchMark |
BenchMark XT | BenchMark GX | Manual/Semi- automated Processors |
CINtec® p16 Histology (Except China, Thailand and Japan) |
06695248001 | 50 | ✔ | ✔ | ✔ | - |
CINtec® p16 Histology (Except China, Thailand and Japan) |
06695256001 | 250 | ✔ | ✔ | ✔ | - |
CINtec® p16 Histology (CE/IVD in China and Thailand; RUO in Japan) |
06695221001 | 50 | ✔ | ✔ | - | - |
CINtec® Histology Kit - CE/IVD, Canada Class II and Japan RUO |
||||||
| System Compatiblity | ||||||
Product Name** |
Material Number |
Number of Tests |
BenchMark ULTRA |
BenchMark XT | BenchMark GX | Manual/Semi- automated Processors |
CINtec® Histology Kit (Except Canada and Japan) |
06594441001 | 50 | - | - | - | ✔ |
CINtec® Histology Kit (Class II in Canada and RUO Japan) |
06594450001 |
50 | - | - | - | ✔ |
* Diagnostics performance by individual pathologists compared with the consensus diagnosis established by expert gynecopathologists using H&E along with CINtec® Histology.
** Please check with your local Roche representative about product availability in your area; some products may not be available in all countries.
References
- Bergeron C, Ordi J, Schmidt D, Trunk MJ, Keller T, Ridder R, et al. Conjunctive p16INK4a Testing Significantly Increases Accuracy in Diagnosing High-Grade Cervical Intraepithelial Neoplasia. Am J Clin Pathol. 2010;133(3):395–406.