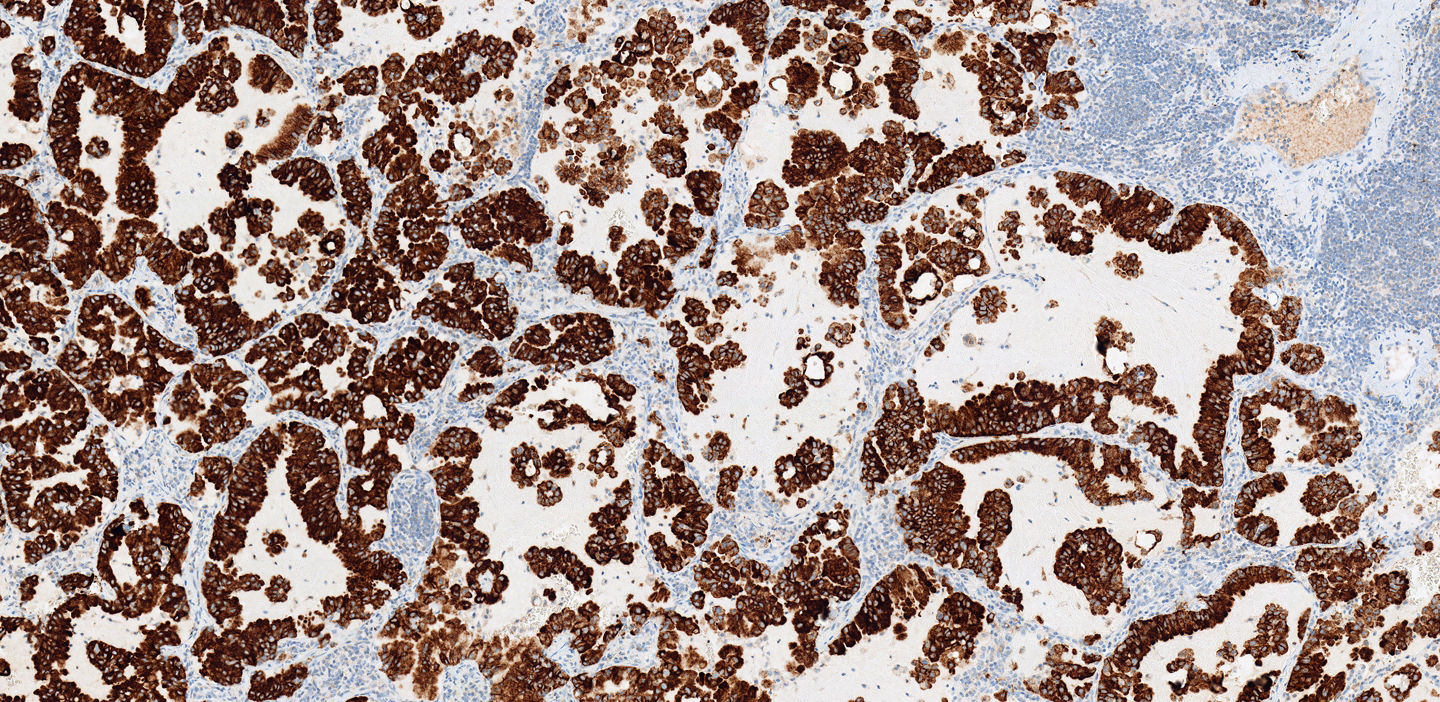
Clinical guidelines recommend routine testing for genetic mutations in all adenocarcinomas, including ALK EML4 gene rearrangement. Testing is recommended immediately after establishing histology and is required prior to initiating targeted therapy for a patient. The current approved methods for testing include IHC and FISH.
VENTANA anti-ALK (D5F3) Rabbit Monoclonal Primary Antibody (CE IVD)

ALK targeted therapy
- VENTANA anti-ALK (D5F3) Rabbit Monoclonal Primary Antibody* stained with OptiView DAB Detection and Amplification detects the ALK protein that is the target of therapy
- Clinical guidelines recommend rapid turnaround for earlier targeted therapies
- ALK has comparable sensitivity and specificity relative to FISH
- Make more immediate treatment decisions for advanced NSCLC patients by using the VENTANA anti-ALK (D5F3) Assay
* VENTANA ALK (D5F3) Assay
Non-small cell lung cancer (NSCLC)
Crizotinib, alectinib and lorlatinib are clinically effective and CE Marked for the treatment of patients with metastatic NSCLC whose tumors are ALK-positive as detected by an FDA-approved testing method for ALK.1, 3, 7
- Crizotinib is indicated for the treatment of patients with ALK positive metastatic NSCLC and other kinases¹
- Ceritinib is indicated for the treatment of patients with ALK-positive metastatic NSCLC who have had no previous treatment, or who have progressed on, or who are intolerant to, crizotinib3
- Alectinib is indicated for the treatment of patients with ALK-positive metastatic NSCLC who have had no previous treatment, or who have progressed on, or who are intolerant to, crizotinib7
- Lorlatinib is indicated for patients with ALK-positive metastatic non-small cell lung cancer (NSCLC) whose disease has progressed on crizotinib and at least one other ALK inhibitor for metastatic disease; or whose disease has progressed on alectinib or ceritinib as the first ALK inhibitor therapy for metastatic disease12
Technical benefits of IHC testing
ALK FISH can present technical challenges in evaluating patient results and offers the potential for false negatives. Recent studies indicate that the VENTANA ALK (D5F3) Assay stained with OptiView DAB Detection and Amplification is sensitive and specific for determination of ALK status, and a better alternative to ALK FISH. There are reports of ALK IHC-positive, FISH-negative patients benefitting from treatment with crizotinib, ceritinib or alectinib. 8,9,10,11
VENTANA ALK (D5F3) Assay and detection with amplification vs. FISH
Se hela tabellenVENTANA ALK (D5F3) Assay and detection with amplification vs. FISH
In one study, van der Wekken et al. “…found that dichotomous ALK-IHC is superior to ALK-FISH on small biopsies and fine-needle aspirations (FNA) to predict tumor response and survival to crizotinib for patients with advanced NSCLC.” 7
In one study, van der Wekken et al. “…found that dichotomous ALK-IHC is superior to ALK-FISH on small biopsies and fine-needle aspirations (FNA) to predict tumor response and survival to crizotinib for patients with advanced NSCLC.” 7
| VENTANA ALK (D5F3) Assay with OptiView DAB Detection and Amplification |
ALK FISH |
|
|---|---|---|
Easy to score |
|
|
| Faster turnaround times |
|
|
| Brightfield vs. fluorescent staining |
|
|
VENTANA anti-ALK (D5F3) Rabbit Monoclonal Primary Antibody (VENTANA anti-ALK (D5F3)) is intended for laboratory use in the detection of the anaplastic lymphoma kinase (ALK) protein in formalin-fixed, paraffin-embedded non-small cell lung carcinoma (NSCLC) tissue stained with the BenchMark IHC/ISH instruments. It is indicated as an aid in identifying patients eligible for treatment with crizotinib, ceritinib, or alectinib or lorlatinib.
This product should be interpreted by a qualified pathologist in conjunction with histological examination, relevant clinical information, and proper controls.
This product is intended for in vitro diagnostic (IVD) use.
ALK targeted therapy
- VENTANA anti-ALK (D5F3) Rabbit Monoclonal Primary Antibody* stained with OptiView DAB Detection and Amplification detects the ALK protein that is the target of therapy
- Clinical guidelines recommend rapid turnaround for earlier targeted therapies
- ALK has comparable sensitivity and specificity relative to FISH
- Make more immediate treatment decisions for advanced NSCLC patients by using the VENTANA anti-ALK (D5F3) Assay
* VENTANA ALK (D5F3) Assay
Non-small cell lung cancer (NSCLC)
Crizotinib, ceritinib and alectinib are clinically effective and indicated for the treatment of patients with metastatic NSCLC whose tumors are ALK-positive as detected by an FDA-approved testing method for ALK.1, 3, 7
- Crizotinib is indicated for the treatment of patients with ALK positive metastatic NSCLC and other kinases¹
- Ceritinib is indicated for the treatment of patients with ALK-positive metastatic NSCLC who have had no previous treatment, or who have progressed on, or who are intolerant to, crizotinib3
- Alectinib is indicated for the treatment of patients with ALK-positive metastatic NSCLC who have had no previous treatment, or who have progressed on, or who are intolerant to, crizotinib7
Technical benefits of IHC testing
ALK FISH can present technical challenges in evaluating patient results and offers the potential for false negatives. Recent studies indicate that the VENTANA ALK (D5F3) Assay stained with OptiView DAB Detection and Amplification is sensitive and specific for determination of ALK status, and a better alternative to ALK FISH. There are reports of ALK IHC-positive, FISH-negative patients benefitting from treatment with crizotinib, ceritinib or alectinib. 8,9,10,11
References
- XALKORI® (crizotinib) [package insert], New York, NY: Pfizer; 2012.
- Ferlay, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., Parkin, D.M., Forman, D., Bray, F. (2012). GLOBOCAN v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 Lyon, France: International Agency for Research on Cancer; 2013. Available at: http://globocan.iarc.fr (last accessed March 2016).
- ZYKADIA (ceritinib) [package insert], Whippany, NJ: Novartis Pharmaceuticals Corporation 2016.
- ALECENSA (alectinib) [package insert], San Francisco, CA: Genentech; 2017.
- World Health Organization. International Agency for Research on Cancer. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Lyon, France http://globocan.iarc.fr/ Default.aspx. Accessed August 1, 2014.
- Lung Cancer Survival Rates and Prognosis. National Cancer Institute at the National Institutes of Health. Bethesda, MD. http://www.cancer.gov/cancertopics/types/lung/cancer-survival-prognosis Accessed August 1, 2014.
- van der Wekken et al Dichotomous ALK-IHC is a better predictor for ALK inhibition outcome than traditional ALK-FISH in advanced non-small cell lung cancer, Clinical Cancer Research, 2017. Available at http://clincancerres.aacrjournals.org/content/early/2017/02/09/1078-0432. CCR-16-1631.
- Zhou J, Zhao J, Sun K, Wang B, Wang L, et al. Accurate and Economical Detection of ALK Positive Lung Adenocarcinoma with Semiquantitative Immunohistochemical Screening. PLoS ONE (2014) 9(3): e92828. doi:10.1371/journal.pone.0092828.
- Ling Shan, Fang Lian, Lei Guo, Xin Yang, Jianming Ying and Dongmei Lin. Combination of conventional immunohistochemistry and qRT-PCR to detect ALK rearrangement. Diagnostic Pathology 2014, 9:3. doi:10.1186/1746-1596-9-3.
- Ying, J.; Guo, L.; Qiu, T.; Shan, L.; Ling, Y.; Liu, X.; Lu, N. Diagnostic value of a novel fully automated immunochemistry assay for detection of ALK rearrangement in primary lung adenocarcinoma. Annals of Oncology. 24(10):2589-2593, October 2 Modern Pathology (2013) 26, 1545–1553; doi:10.1038/modpathol.2013.87; published online 7 June 2013.
- Mok T, Peters S, Camidge DR. Patients with ALK IHC-positive/fish-negative NSCLC benefit from ALK TKI treatment: response data from the global ALEX trial. Presented at: the IASLC 18th World Conference on Lung Cancer; October 15-18; Yokohama, Japan. Poster MA 07.01
- LORVIQUA (lorlatinib) [package insert]. Bruxxels: Pfizer Europe MA EEIG; 2022.