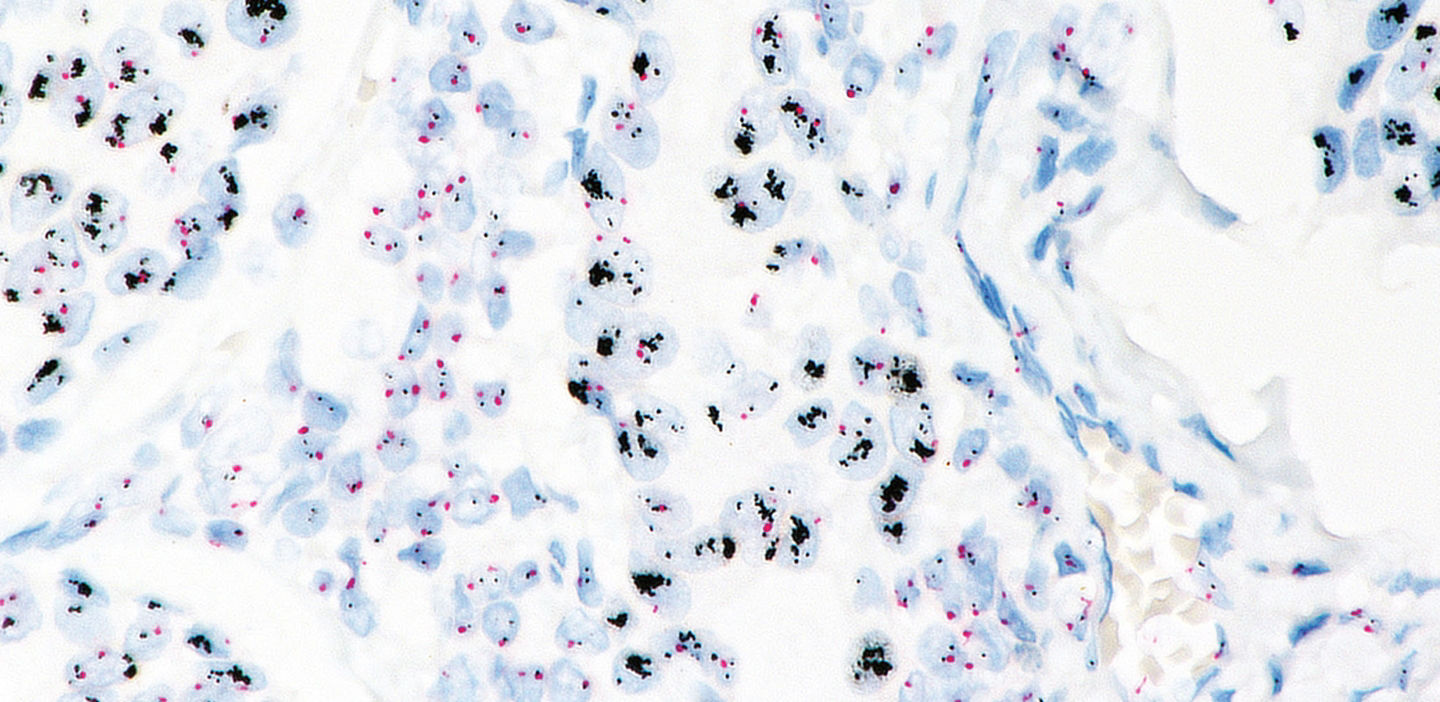
VENTANA HER2 Dual ISH DNA Probe Cocktail assay (CE IVD)

Optimized with new oligo probes, the VENTANA HER2 Dual ISH assay delivers clear, confident reads
The new VENTANA HER2 Dual ISH DNA Probe Cocktail assay is a fully automated, ready-to-use brightfield solution for determining HER2 gene status. VENTANA HER2 Dual ISH helps identify breast and gastric cancer patients eligible for treatment with HER2-targeted personalized therapies.
Increased performance
- Oligo probes and new detection kits
- High first pass rates1
Easily interpreted using brightfield microscopy2
- Allows for interpretation within the context of tissue morphology
- Identifies tumour heterogeneity
- Produces an archivable result
Robust & reproducible
- Highly concordant with FISH1
- Highly reproducible between laboratories & pathologists1
Diagnostic confidence
- Fully automated solution with fast turnaround time
- Widely adopted breast cancer portfolio with high clinical utility
Importance of HER2 in oncology - breast and gastric cancers

Breast cancer comprises 24% of all female cancers and is estimated to claim the lives of more than 600,000 globally each year.3

There is growing evidence that HER2 is an important biomarker and key driver of tumorigenesis in gastric cancer, with studies showing amplification or overexpression in 7–34% of tumours.5

15-20% of breast cancers overexpress HER2 and are eligible for HER2-targeted therapies, which are proven to improve outcomes.4

Demonstration of HER2 gene amplification and/or protein overexpression is essential for selecting patients for trastuzumab therapy.

Gastric cancer has the third highest mortality rate, with more than 750,000 deaths per year.3
Deliver diagnostic confidence
Multiple guidelines, including NCCN and CAP/ASCO6,7, recommend the evaluation of human epidermal growth factor receptor 2 (HER2) protein expression by immunohistochemistry (IHC) assay for breast cancer specimens. Depending on the IHC result, a reflex test should be performed-using in situ hybridization (ISH).
The VENTANA HER2 Dual ISH DNA Probe Cocktail is intended to determine HER2 gene status by enumeration of the ratio of the HER2 gene to Chromosome 17 by light microscopy. This ready-to-use assay is optimized for use on formalin-fixed, paraffin-embedded human breast and gastric carcinoma tissue specimens with the VENTANA Silver ISH DNP Detection Kit and the VENTANA Red ISH DIG Detection Kit, on the fully-automated BenchMark IHC/ISH instruments.
The VENTANA HER2 Dual ISH DNA Probe Cocktail is indicated as an aid in the assessment of patients for whom trastuzumab is being considered.
Why brightfield is better
- No need for fluorescent microscope/oil and darkroom
- Fits in pathologists’ regular workflow at brightfield scope
- Can be read alongside/at same time as H&E and other breast panel markers on the same case for easy comparison and correlation of findings
- No interference from tissue auto fluorescence or tissue marking dyes
- Avoids need for oil immersion lens
Provides morphological context for the pathologist
- Signals visualized at lower magnification, allowing pathologist to easily scan the entire tissue section to identify invasive carcinoma and tumour heterogeneity
- Counterstained with Hematoxylin II like IHC slides so morphologic features are preserved and visible. Allows distinction between invasive and in situ carcinoma, identification of normal cells and easy comparison to H&E slide and IHC slides
- Normal cells provide a same slide internal control of the staining process so you can be confident of the results
- Archivable results
- Signals do not fade over time
References
- Ventana Product Document Library. Package Insert, VENTANA HER2 Dual ISH DNA Probe Cocktail.
- Ventana Product Document Library. Interpretation Guide for VENTANA HER2 Dual ISH DNA Probe Cocktail assay; Staining for Breast and Gastric Carcinoma.
- Globocan 2018. Global Cancer Observatory, International Agency for Research on Cancer (http://gco.iarc.fr/).
- Wolff AC, Hicks D, Hammond E. ASCO/CAP Clinical Practice Guideline Update. J Clin Oncol.2013;31(31):3997-4013.
- Bang YJ, Van Cutsem E, Feyereislova A, et al: ToGA Trial Investigators: Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376: 687-697
- Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. J Clin Oncol. 2018 Jul 10;36(20):2105-2122.
- NCCN Guidelines Version 1.2018. NCCN Clinical Practice Guidelines in Oncology Breast Cancer. NCCN.org.
The VENTANA HER2 Dual ISH DNA Probe Cocktail is intended to determine HER2 gene status by enumeration of the ratio of the HER2 gene to Chromosome 17 by light microscopy. The HER2 and Chromosome 17 probes are detected using two-color chromogenic in situ hybridization (ISH) in formalin-fixed, paraffin-embedded human breast and gastric carcinoma tissue specimens, including the gastroesophageal junction, following staining on BenchMark IHC/ISH instruments.
The VENTANA HER2 Dual ISH DNA Probe Cocktail is indicated as an aid in the assessment of patients for whom trastuzumab is being considered. This product should be interpreted by a qualified pathologist in conjunction with histological examination, relevant clinical information, and proper controls.
This product is intended for in vitro diagnostic (IVD) use.
VENTANA Silver ISH DNP Detection Kit
The VENTANA Silver ISH DNP Detection Kit is an indirect system for detecting DNP-labeled targets. The kit is intended to identify targets by silver in situ hybridization (ISH) in sections of formalin-fixed, paraffin-embedded tissue that are stained on BenchMark IHC/ISH instruments.
This product should be interpreted by a qualified reader in conjunction with histological examination, relevant clinical information, and proper controls.
This product is intended for in vitro diagnostic (IVD) use.
VENTANA Red ISH DIG Detection Kit
The VENTANA Red ISH DIG Detection Kit is an indirect system for detecting DIG-labeled targets. The kit is intended to identify targets by chromogenic red in situ hybridization (ISH) in sections of formalin-fixed, paraffin-embedded tissue that are stained on BenchMark IHC/ISH instruments.
This product should be interpreted by a qualified reader in conjunction with histological examination, relevant clinical information, and proper controls.
This product is intended for in vitro diagnostic (IVD) use.
Ordering Information
Se hela tabellenOrdering Information
Ordering codes for VENTANA HER2 Dual ISH Probe Cocktail and related products
Ordering codes for VENTANA HER2 Dual ISH Probe Cocktail and related products
| Product | Catalog Number |
Ordering Code |
Test |
|---|---|---|---|
| VENTANA HER2 Dual ISH DNA Probe Cocktail |
800-6043 |
08314373001 |
30 |
| VENTANA Silver ISH DNP Detection Kit |
760-516 |
08318883001 |
60 |
| VENTANA Red ISH DIG Detection Kit |
760-512 |
08318832001 |
60 |