LumiraDx and cobas® liat analyser provide a comprehensive portfolio to aid you in the differential diagnosis of SARS-CoV-2, Influenza A/B and RSV.9-15
Roche Respiratory Solutions in the UK and Ireland
Helping you transform what's possible with respiratory testing
Roche Respiratory Solutions can assist experts in diagnosing, correctly treating, and discharging patients faster, potentially easing the burden on healthcare professionals and helping to reduce winter pressures.
Rapid respiratory test results help empower clinicians to deliver treatment at the earliest possible stage – potentially reducing the risk of escalation and improving patient outcomes.
The Roche solution includes tests, technologies and transformation support for introducing rapid respiratory testing into any clinical setting - bringing care closer to the patient.
Enhancing care across all pathways
Our respiratory solutions are proven to deliver accurate diagnostics where they are needed most; in the community, in clinics, in hospitals, and helping to protect high-risk patients.

Respiratory Testing in Primary Care
Quick, targeted near-patient respiratory testing in the community, clinics and point of entry.
The primary care setting plays a critical role in the diagnosis and management of respiratory infections, with respiratory symptoms being the most common reason for primary care consultations.1

LumiraDx
LumiraDx is designed to make testing easy, efficient and more accessible.
Portable battery-operated system
Rapid results in 12 minutes or less
- Additional assays beyond respiratory

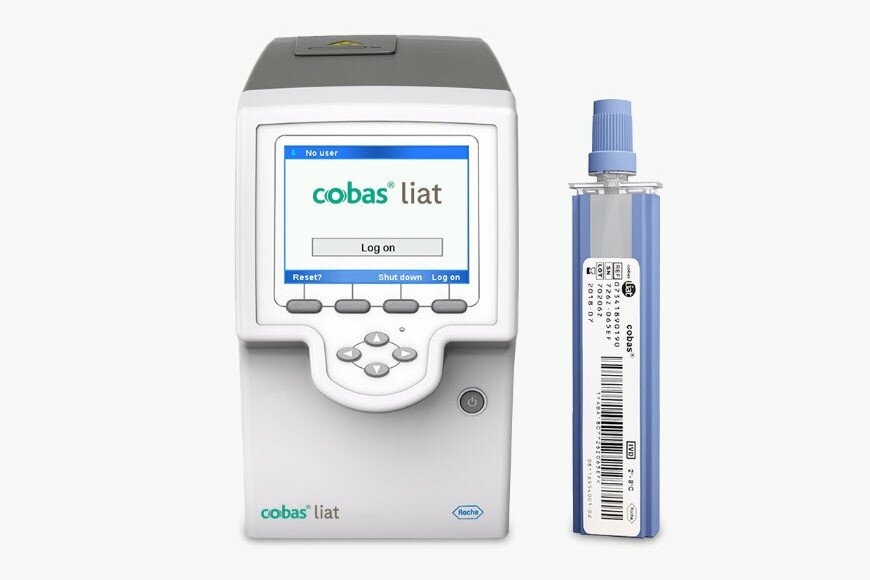
cobas® liat analyser
The cobas® liat analyser delivers gold-standard PCR testing at the point of care.
Quick, targeted near-patient testing
Actionable results in 20 minutes.
Frontline multiplex assays
Benefits of rapid respiratory testing in primary care
For a new testing pathway to be adopted at local, regional or national level, it will have to show benefits for the patient, the clinical team and the healthcare system. Rapid respiratory point of care testing in primary care may help to improve diagnosis, clinician confidence, and patient satisfaction.

Rapid diagnosis and treatment
The availability of rapid diagnostic results may increase clinicians' confidence in the diagnosis and enable them to offer appropriate advice, guidance, and treatment at the right time and place.3,4,7

Clinical decision support
Empowering doctors with on-the-spot insights to differentiate bacterial from viral infections may help improve prescribing accuracy and thereby reduce inappropriate antibiotic prescribing.4,5

Fewer secondary care referals
Point of Care testing may help reduce unnecessary referrals, potentially streamlining patient management, alleviating pressure on secondary care and improving patient satisfaction.6,8

Potential cost-saving efficiencies
Appropriate use of Point of Care testing facilitates early diagnosis and treatment that may lead to better outcomes, thereby reducing need for secondary care and helping reduce overall cost of care.6,7
Developing rapid respiratory testing for primary care settings
Access to diagnostics in the community can be powerful. We know that getting a diagnosis quickly can help patients receive effective treatment, but this doesn’t always happen.
Roche has been involved in several pilots to assess the impact of rapid respiratorytesting for primary care settings, bringing testing closer to patients.

Flu Test & Treat Community Pathway
Explore how a flu patient community pathway pilot in Yorkshire and the North West achieved significant results with point-of-care testing. This infographic highlights the project's key outcomes, including estimated reductions in hospital admissions and ICU stays. Download the infographic to learn more about the initiative's impact on healthcare practices.

Acute Respiratory Hub Pathway Evaluation
Discover the benefits of integrating rapid point of care tests in Acute Respiratory Infection (ARI) hubs. Our infographic provides valuable insights on how diagnostic results influence prescribing decisions and patient management, based on a pilot evaluation conducted in partnership with Copeland Primary Care Network. Download the infographic to learn more.

A Rapid Flu Test-And-Treat Community Pathway
A rapid ‘test and treat’ influenza (flu) community pathway incorporating a rapid diagnostic point of care test (POCT) was found to enhance clinical decision-making and thus reduce unnecessary antibiotic use and enable timely antiviral treatment to decrease the impact of infectious diseases and antimicrobial resistance. Download publication to learn more.
Featured products

You might be interested in

Dr Chris Turner explores the evidence around the benefits and impact of near patient testing in primary care. Evidence shows point of care testing (POC) is a cost-effective way to diagnose patients faster than ever, helping empower doctors with the diagnostic confidence to make the best treatment decisions for each patient.
Dr Chris Turner explores the evidence around the benefits and impact of near patient testing in primary care. Evidence shows point of care testing (POC) is a cost-effective way to diagnose patients faster than ever, helping empower doctors with the diagnostic confidence to make the best treatment decisions for each patient.

Managing respiratory infections affects everyone who relies on healthcare systems.
Managing respiratory infections affects everyone who relies on healthcare systems.

A health service transformation expert, Sheila helped lead several point-of-care respiratory testing pilots across the UK.
A health service transformation expert, Sheila helped lead several point-of-care respiratory testing pilots across the UK.

Respiratory illnesses – including seasonal flu epidemics, RSV, Strep A and COVID-19 (SARS-CoV-2) – pose considerable challenges to NHS primary and secondary care providers.1
Respiratory illnesses – including seasonal flu epidemics, RSV, Strep A and COVID-19 (SARS-CoV-2) – pose considerable challenges to NHS primary and secondary care providers.1
Respiratory Testing in Hospital Wards & Point of Entry
Fast, reliable and robust respiratory testing.
Every hospital admission in winter - whether through A&E or onto a ward.

LumiraDx
LumiraDx is designed to make testing easy, efficient and more accessible.
Portable battery-operated system
Rapid results in 12 minutes or less
- Additional assays beyond respiratory

cobas® liat analyser
The cobas® liat analyser delivers gold-standard PCR testing at the point of care.
Quick, targeted near-patient testing
Actionable results in 20 minutes.
Frontline multiplex assays
LumiraDx and cobas® liat analyser provide a comprehensive portfolio to aid you in the differential diagnosis of SARS-CoV-2, Influenza A/B and RSV.9-15
Targeted near-patient testing

Rapid results

Additional assays

Featured products


You might be interested in

Roche has developed a comprehensive range of rapid respiratory tests, technologies and assays for primary, secondary and community care settings. We are helping the NHS to transform what’s possible with respiratory testing.
Roche has developed a comprehensive range of rapid respiratory tests, technologies and assays for primary, secondary and community care settings. We are helping the NHS to transform what’s possible with respiratory testing.

Managing respiratory infections affects everyone who relies on healthcare systems.
Managing respiratory infections affects everyone who relies on healthcare systems.

Respiratory illnesses – including seasonal flu epidemics, RSV, Strep A and COVID-19 (SARS-CoV-2) – pose considerable challenges to NHS primary and secondary care providers.1
Respiratory illnesses – including seasonal flu epidemics, RSV, Strep A and COVID-19 (SARS-CoV-2) – pose considerable challenges to NHS primary and secondary care providers.1
Respiratory Testing in Hospitals
High-throughput molecular diagnostic testing.

Roche cobas® 5800, 6800 and 8800 systems aim to consolidate up to 90% of routine molecular testing into a single workflow,2 enabling under-pressure lab staff to focus on adding value in other areas.
Roche’s advanced cobas® Respiratory flex is a flexible, high-throughput, syndromic test that can be customised by clinicians. They can order respiratory tests that are optimised for settings and address seasonality, local dynamics and patient cohort.
Flexible syndromic testing

High-throughput multiplex

Reliable and robust

Featured products



You might be interested in

Roche has developed a comprehensive range of rapid respiratory tests, technologies and assays for primary, secondary and community care settings. We are helping the NHS to transform what’s possible with respiratory testing.
Roche has developed a comprehensive range of rapid respiratory tests, technologies and assays for primary, secondary and community care settings. We are helping the NHS to transform what’s possible with respiratory testing.

Managing respiratory infections affects everyone who relies on healthcare systems.
Managing respiratory infections affects everyone who relies on healthcare systems.

Respiratory illnesses – including seasonal flu epidemics, RSV, Strep A and COVID-19 (SARS-CoV-2) – pose considerable challenges to NHS primary and secondary care providers.1
Respiratory illnesses – including seasonal flu epidemics, RSV, Strep A and COVID-19 (SARS-CoV-2) – pose considerable challenges to NHS primary and secondary care providers.1

Introducing the cobas® Respiratory flex assay, designed to enhance your diagnostic capabilities with Roche-developed innovative TAGS (temperature activated generation of signal) technology.
Introducing the cobas® Respiratory flex assay, designed to enhance your diagnostic capabilities with Roche-developed innovative TAGS (temperature activated generation of signal) technology.
Respiratory Testing for High-Risk Patients
Precision respiratory testing.

In complex cases, clinicians need diagnostic confidence to help enable them to select the most appropriate treatment and make informed prescribing decisions.
Roche cobas® eplex can perform rapid syndromic diagnosis, testing a single sample for 20 conditions and delivering a result in 90 minutes.
Tests 20+ respiratory targets

Easy to use workflow

Urgent results for high-risk patients

Featured products

References
1. Abdel-Aal A et al. Prioritising primary care respiratory research needs: results from the 2020 International Primary Care Respiratory Group (IPCRG) global e-Delphi exercise. NPJ Prim Care Respir Med. 2022 Jan 28;32:6.
2. (2024). Roche. Available at: https://diagnostics.roche.com/global/en/products/instruments/cobas-8800-ins-2694.html (Accessed: 2 October 2024).
3. Cooke J, et al. BMJ Open Resp Res. 2015;2:e000086.
4. Lingervelder D, et al. Int J Clin Pract. 2019;73:e13392.
5. Thornton HV, et al. British Journal of General Practice. 2020;70(701):574-575.
6. Schols AMR, et al. British Journal of General Practice. 2018;68(673):362-363.
7. Mills SEE, et al. BJGP Open. 2024;8(2):BJGPO.2023.0094.
8. Turner PJ, et al. Family Practice. 2016;33(4):388–394.
9. LumiraDx Product Insert SARS-CoV-2 Ag (version 14, 2022)
10. LumiraDx Product Insert SARS-CoV-2 Ag Ultra (version 1, 2022-07)
11. LumiraDx Product Insert SARS-CoV-2 & Flu A/B (version 5, 2022)
12. LumiraDx Product Insert SARS-CoV-2 & RSV (version 3, 2023-08)
13. cobas® Influenza A/B & RSV Product Insert
14. cobas® SARS-C0V-2 Product Insert
15. cobas® SARS-C0V-2 & Influenza A/B Product Insert