For localized information and support, would you like to switch to your country-specific website for {0}?
VENTANA® MMR RxDx Panel

The VENTANA MMR RxDx Panel is the first and only immunohistochemistry-based assay panel approved to identify endometrial carcinoma patients eligible for treatment with JEMPERLI (dostarlimab). Endometrial cancer (EC) is the most common gynaecological malignancy in the U.S. and the fourth most common cancer in women in North America.1,2 It has the highest rates of mismatch repair (MMR) deficiency of all tumors (20-40%).3,4,5 There are limited treatment options for women whose disease progresses on or after first-line therapy.6
What is mismatch repair (MMR)?
DNA mismatch repair is a system for recognizing and repairing errors that can spontaneously occurs during DNA replication. When this mismatch repair mechanism isn’t working properly, it is most commonly attributed to mutations in the MMR proteins, MLH1, PMS2, MSH2, and MSH6.


MMR deficiency serves as a predictive biomarker for a new immunotherapeutic option for patients.7
Multiple studies have demonstrated that MMR deficiency correlates with higher expression of PD-1 or PD-L1, possibly due to increased neoantigen expression associated with tumor mutation burden that results from replication errors.1,8 Thus, MMR proteins may be useful as predictive biomarkers for PD-1 targeted therapy; specifically, a loss of expression of one or more MMR proteins might predict an increased likelihood of response to such therapy.9, 10, 11
Intended use
VENTANA MMR RxDx Panel is a qualitative immunohistochemistry test intended for use in the assessment of mismatch repair (MMR) proteins (MLH1, PMS2, MSH2 and MSH6) in formalin-fixed, paraffin-embedded (FFPE) endometrial carcinoma tissue by light microscopy. The OptiView DAB IHC Detection Kit is used for MSH6, MSH2 and MLH1, and the OptiView DAB IHC Detection Kit with the OptiView Amplification Kit is used for PMS2 on a VENTANA BenchMark ULTRA instrument.
VENTANA MMR RxDx Panel is indicated as an aid in identifying patients eligible for treatment with therapies listed in Table 1 for the respective indications and status in accordance with the approved therapeutic product labeling.
Table 1. VENTANA MMR RxDx Panel companion diagnostic indications
View full tableTable 1. VENTANA MMR RxDx Panel companion diagnostic indications
| Indication for use | Therapy |
Status |
|---|---|---|
Endometrial Carcinoma (EC) |
JEMPERLI (dostarlimab) | Deficient MMR (dMMR) |
Panel interpretation
A loss of expression of any of the essential MMR proteins, including MLH1, PMS2, MSH2, or MSH6, in the presence of evaluable internal controls indicates MMR deficiency. Presence of staining for all four MMR protein markers in the tumor in the presence of evaluable internal controls indicates that the case is proficient for mismatch repair status. Absence of staining in any one of the MMR protein markers of VENTANA MMR RxDx Panel indicates a case is deficient for mismatch repair status.
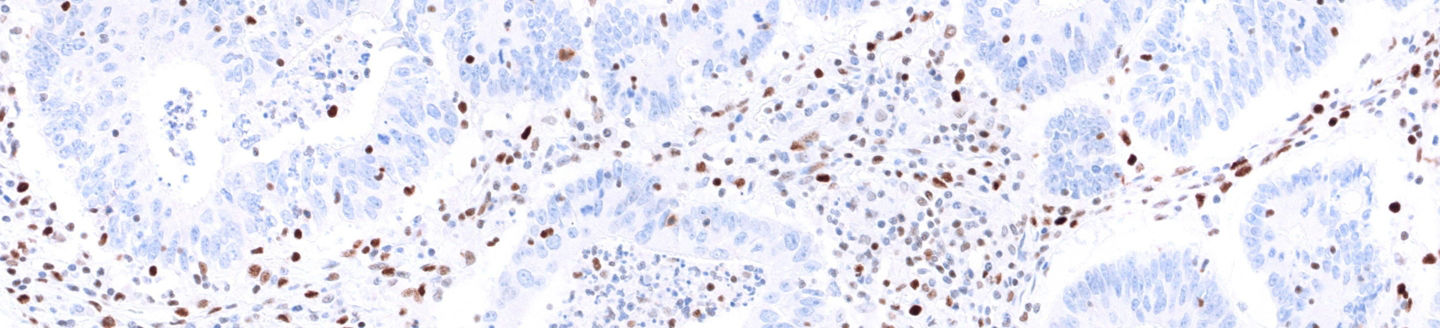
Case 1: VENTANA anti-MLH1 (M1) Mouse Monoclonal Primary Antibody staining
Figure 1: VENTANA anti-MLH1 (M1) Mouse Monoclonal Primary Antibody staining with Intact (left) or Loss (right) of expression in the presence of evaluable internal controls in endometrial carcinoma tissue.

Case 3: VENTANA anti-MSH2 (G219-1129) Mouse Monoclonal Primary Antibody staining
Figure 3: VENTANA anti-MSH2 (G219-1129) Mouse Monoclonal Primary Antibody staining with Intact (left) or Loss (right) of expression in the presence of evaluable internal controls in endometrial carcinoma tissue.

Case 2: VENTANA anti-MSH6 (SP93) Rabbit Monoclonal Primary Antibody staining
Figure 2: VENTANA anti-MSH6 (SP93) Rabbit Monoclonal Primary Antibody staining with Intact (left) or Loss (right) of expression in the presence of evaluable internal controls in endometrial carcinoma tissue.

Case 4: VENTANA anti-PMS2 (A16-4) Rabbit Monoclonal Primary Antibody staining
Figure 4: VENTANA anti-PMS2 (A16-4) Rabbit Monoclonal Primary Antibody staining with Intact (left) or Loss (right) of expression in the presence of evaluable internal controls in endometrial carcinoma tissue.

About the VENTANA MMR IHC Panel
The VENTANA MMR IHC Panel was launched in 2017 as an aid in the identification of Lynch syndrome in patients diagnosed with colorectal cancer. In addition to the same four MMR proteins listed above (MLH1, MSH2, MSH6 and PMS2), the VENTANA MMR IHC Panel also includes VENTANA BRAF V600E (VE1) Mouse Monoclonal Antibody. The combination of these five antibodies are used clinically to aid in the identification of Lynch syndrome, which is a genetic predisposition for colorectal and other cancers.
The antibodies in the VENTANA MMR RxDx Panel are the same as those included in the VENTANA MMR IHC Panel, except for VENTANA BRAF V600E (VE1) - same part numbers, same package inserts, but with two separate intended uses. By keeping the part numbers for the antibodies the same, this offers the opportunity for customers to more easily utilize the additional indication.
References
- Yamashita H, Nakayama K, Ishikawa M, et al. Microsatellite instability is a biomarker for immune checkpoint inhibitors in endometrial cancer. Oncotarget. 2017:9(5):5652-5664.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019:69(1):7-34.
- Kato M, Takano M, Miyamoto M, et al. DNA mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol. 2015:26(1):40-45.
- Matthews KS, Estes JM, Conner MG, et al. Lynch syndrome in women less than 50 years of age with endometrial cancer. Obstet Gynecol. 2008:111(5):1161-6.
- Kim SR, Pina A, Albert A, et al. Does MMR status in endometrial cancer influence response to adjuvant therapy? Gynecol Oncol. 2018:151(1):76-81.
- Lee YC, S Lheureux, and AM Oza. (2017) Treatment strategies for endometrial cancer: current practice and perspective. Curr Opin Obstet Gynecol. 29, 47-58.
- Le et al., Science 357, 409-413 (2017).
- Xiao X, Dong D, He W, et al. Mismatch repair deficiency is associated with MSI phenotype, increased tumor-infiltrating lymphocytes and PD-L1 expression in immune cells in ovarian cancer. Gynecol Oncol. 2018;149(1):146-154.
- Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409-413.
- Sloan EA, Ring KL, Willis BC, et al. PD-L1 expression in mismatch repair-deficient endometrial carcinomas, including Lynch syndrome-associated and MLH1 promoterhypermethylated tumors. Am J Surg Pathol. 2017;41(3):326-333.
- Dudley JC, Lin MT, Le DT, et al. Microsatellite instability as a biomarker for PD-1 blockade. Clin Cancer Res. 2016;22(4):813-820.1. Lortet-Tieulent J, J Ferlay, F Bray, and A Jemal. (2018) International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst. 110, 354-361.