cobas® SARS-CoV-2 & Influenza A/B

Reduce the risk of a misdiagnosis with an accurate nucleic acid test you can trust
Symptoms of COVID-19 and influenza may look the same. It can be difficult for clinicians to identify based on signs & symptoms alone and if left undiagnosed, may result in health complications or community spread.
The cobas® SARS-CoV-2 & Influenza A/B test for use on the cobas® Liat® System provides urgent, accurate answers to rule-in or rule-out influenza and COVID-19 in 20 minutes from a single test, enabling early and effective patient care.
cobas® SARS-CoV-2 & Influenza A/B:
- One sample, one test, 20 minutes, know if it’s COVID-19 or Flu
- Take the workload out of testing with simple intuitive user handling
- Minimize cross-contamination and risk of exposure during testing with the closed-system design
Your priority is your patients. Enable the answers you need to support efficient, on-demand patient care. The cobas® liat system enables definitive and rapid diagnosis, combining powerful PCR technology with fast turnaround time, 20 minutes or less, allowing sample to answer while patients wait.
cobas® SARS-CoV-2 & Influenza A/B performance1
View full tablecobas® SARS-CoV-2 & Influenza A/B performance1
| Target | Positive Agreement | Negative Agreement | LoD |
|---|---|---|---|
| SARS-CoV-2* | 95.3% | 99.4% | 1.2 x 10-2 TCID 50/mL |
| Influenza A** | 96.8% | 99.6% | 2 x 10-3 – 2 x 10-2 TCID 50/mL |
| Influenza B** | 100% | 100% | 2 x 10-3 – 4 x 10-3 TCID 50/mL |
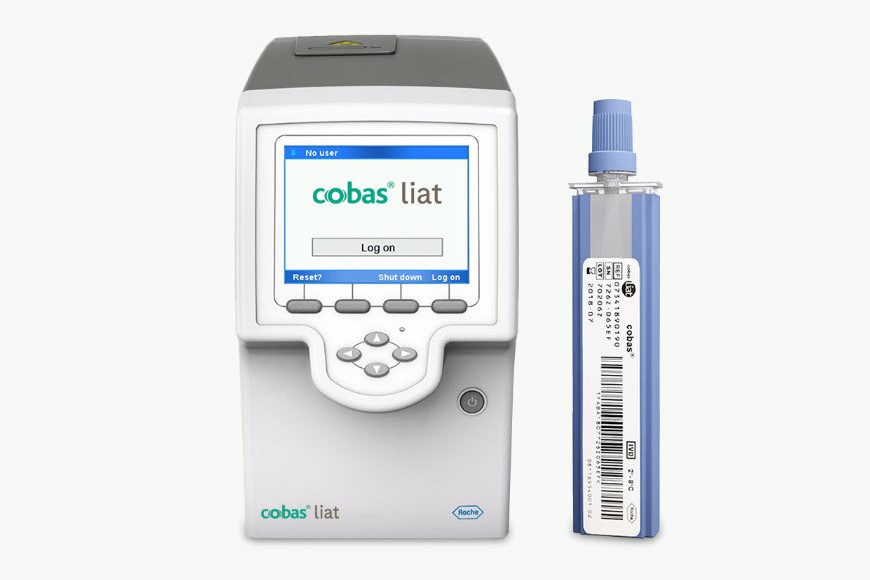
cobas® liat system
Rapidly test, triage, and treat your patients with the accuracy, simplicity, and security needed at the point of care.
Learn moreRegistration status
CE-IVD, FDA 510(k) cleared, CLIA waived
The cobas® liat system is commercially available in select markets. This product is not registered as an in vitro diagnostic (IVD) in all countries; additional information may be available from your Roche sales representative.
Package inserts
Access package inserts through your country’s Roche Diagnostics Website.
References
- cobas® SARS-CoV-2 & Influenza A/B [package insert V05]. Pleasanton, CA: Roche Molecular Systems, Inc., 2023.
COBAS and LIAT are trademarks of Roche.
?wid=1920&hei=768&fmt=png-alpha&fit=crop,1)
