Roche’s FDA-cleared biomarkers represent all three main Alzheimer’s pathologies – amyloid plaques, tau tangles and neurodegeneration. With these biomarkers, identification of AD pathology in early disease stages (as early as mild cognitive impairment) when amyloid targeting therapies are most effective, is possible.1,2
The Elecsys AD CSF ratios have high concordance with β‑Amyloid positron emission tomography (PET) scan results.¹ The Elecsys assays combine both Abeta42 and tau proteins to give accurate and reliable results that can confirm amyloid pathology in early stages of the disease1,3 and aid in Alzheimer’s diagnosis. Compared to a PET scan imaging test in some geographies, these assays are more accessible and cost effective, meaning an accelerated and earlier path to a confirmed diagnosis is within reach for more people.
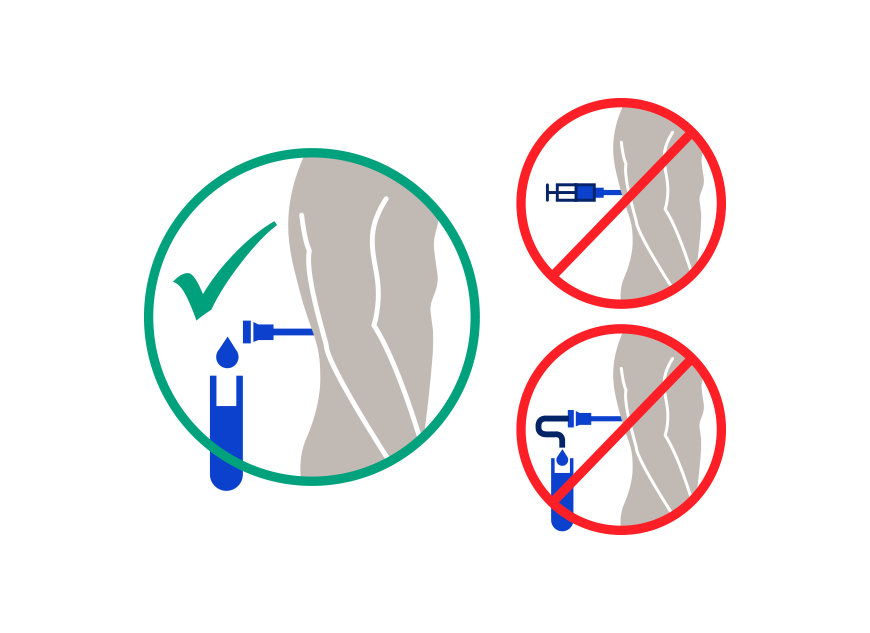
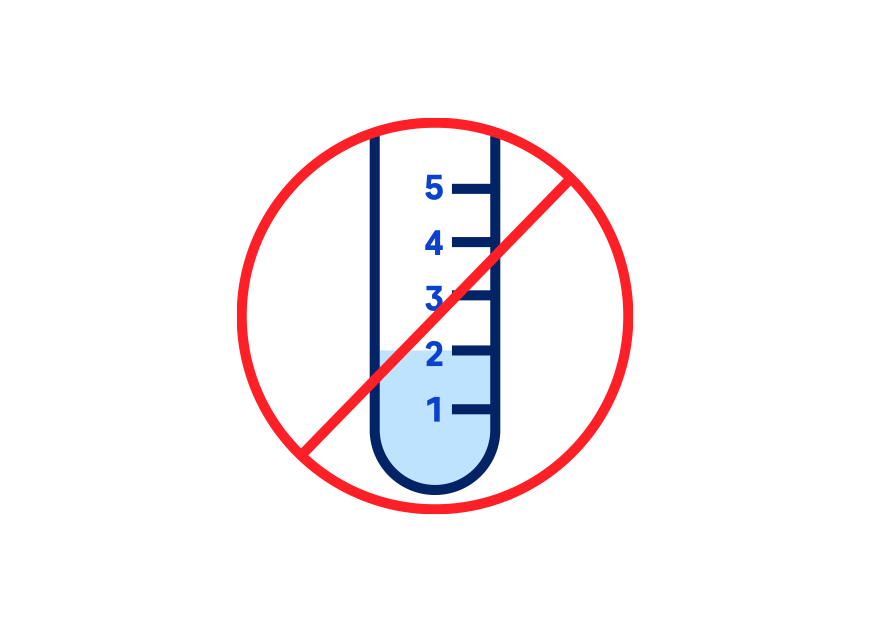
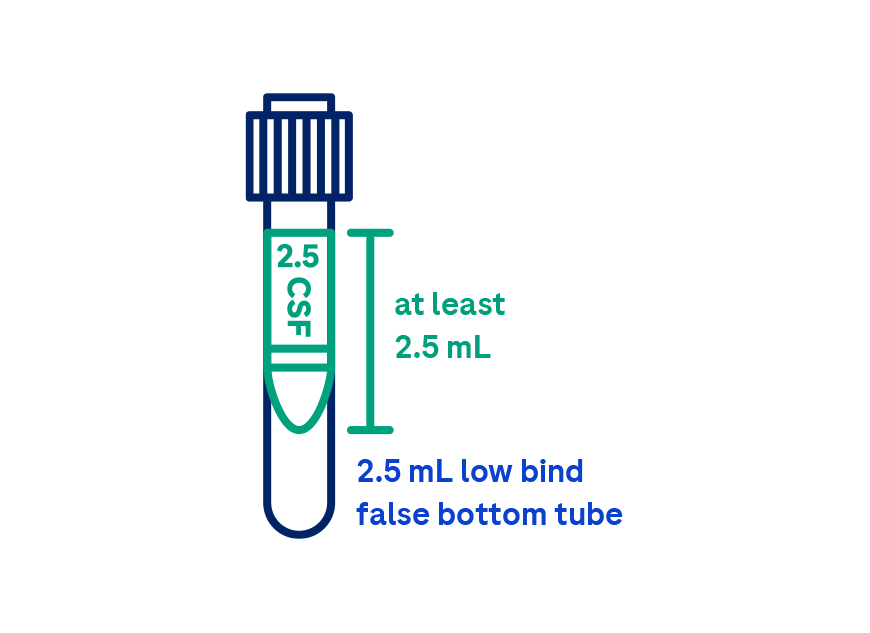
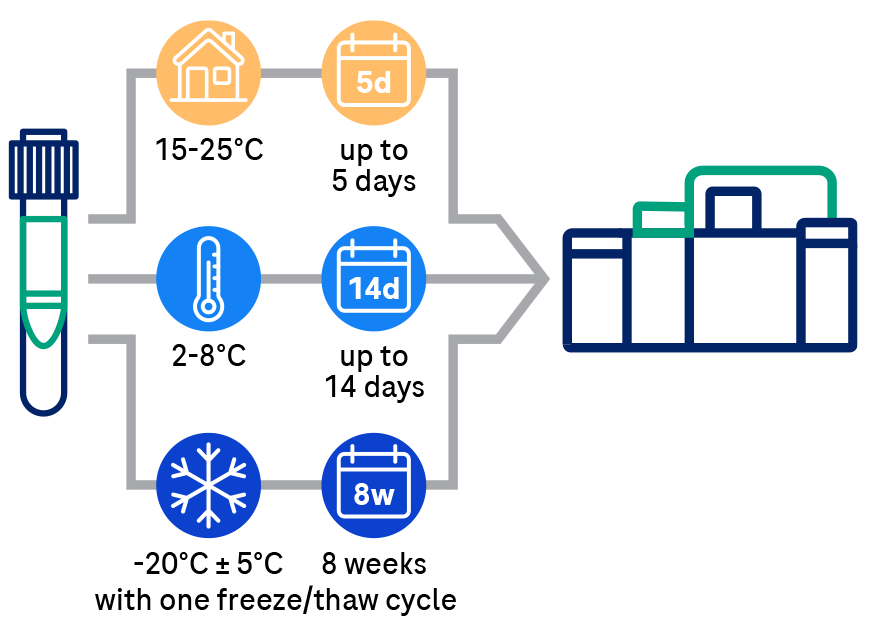
Ordering the Elecsys CSF biomarkers requires careful adherence to pre-analytical sample handling procedures, which are based on recommendations from the Alzheimer’s Association International guidelines1,4. The Abeta42 biomarker, is highly influenced by pre-analytical factors such as CSF collection and sample handling. Extra precautions for sample handling prior to testing are required to avoid Abeta42 loss. With this in mind, it’s important to contact your clinical laboratory partners to gather important ordering, collection, handling and transport instructions. Your lab will be able to provide you with the assay package insert and additional guidance, and in some cases also with the appropriate collection tubes.