Cardiovascular disease is the leading cause of death in the United States for both men and women.1 And in 2021, cardiovascular complications were responsible for one in every five deaths, totaling nearly 700,000.1 In addition to this significant human toll, these complications place an economic burden on the nation, costing nearly $240 billion annually.1
With cardiometabolic multimorbidity becoming increasingly common, cardiometabolic risk factors such as obesity, diabetes, lack of exercise and excess alcohol consumption are contributing to the rise of conditions such as hypertension, dyslipidemia, chronic kidney disease, coronary heart disease, stroke and heart failure.2 Access to cardiometabolic diagnostic tests is crucial, with early detection and intervention essential to preventing complications and disease progression.
Advancement of cardiac biomarkers
As a tool first used to diagnose acute heart failure in the emergency department, cardiac biomarkers have evolved over the past two decades.
Today, cardiac biomarkers are essential in treating and improving care for people with cardiometabolic disease, and are used for both early identification or last-minute supporting intervention. Roche has 20 years of leveraging cutting-edge biomarker technology in cardiometabolic disease diagnosis.

Our two biomarkers include Elecsys® proBNP II and Elecsys® Troponin T Gen 5:
Elecsys proBNP II: Delivering confidence when managing heart failure
Elecsys Troponin T Gen 5: Enabling fast diagnosis of acute myocardial infarction
As cardiac biomarkers and digital solutions evolve, we are committed to continuing research in cardiometabolic disease to advance early diagnosis and help improve clinical decisions.
Lab implications for cardiac biomarkers
In light of recent studies and updated guidelines, laboratories can likely expect an increased volume of cardiac biomarker tests. Laboratorians play a pivotal role, providing critical insights for patients, ranging from those experiencing a cardiac event to individuals undergoing routine testing.
The Safety, Tolerability, and Efficacy of Rapid Optimization of Heart Failure (STRONG-HF) study published in 2022 demonstrated the significant role of the NT-proBNP cardiac biomarker test in improving outcomes for individuals hospitalized due to heart failure. And the 2024 update of the American Diabetes Association's Standards of Care in Diabetes now advocates for the use of the NT-proBNP biomarker test for annual heart failure testing.
References
- CDC.gov. Last accessed February 9, 2024.
- Cheng X, et al., Int J Environ Res Public Health. 2022 Apr 14;19(8):4726.
STRONG-HF clinical study recommends monitoring heart failure with biomarkers
There’s currently no cure for heart failure,1 but to diagnose and monitor it, the n-terminal pro b-type natriuretic peptide (NT-proBNP) biomarker can help.2
A groundbreaking study using NT-proBNP is shifting how people living with heart failure can be cared for. The Safety, Tolerability and Efficacy of Rapid Optimization of Heart Failure (STRONG-HF) study was a multinational, open-label, randomized clinical trial. The study focused on acute heart failure patients who were recently hospitalized for heart failure.
The study found that if a healthcare professional takes a holistic approach, incorporating rapid up-titration of guideline-directed medical therapy (GDMT), regular medical visits, and frequent or serial cardiac biomarker testing (NT-proBNP), it resulted in reduced hospitalizations, lowered risk of death and improvement in patient-reported health status.2
In fact, the STRONG-HF study’s findings were so convincing the Data Safety Monitoring Board stopped the study before it was complete – after 1,000 of the 1,800 patients who took part had at least 90 days of follow-up care because it was considered a significant disadvantage for the control group who received normal treatment. Before this study was published, there was no randomized, controlled clinical trial showing that rapid, simultaneous uptitration of all pillars of GDMT was both safe and effective with the proposed protocol. The STRONG-HF clinical trial provides the highest level of scientific evidence to show how heart failure care can be optimized after an acute event.
STRONG-HF clinical study highlights²
.png)
Significant 34% relative risk reduction and significant 8% absolute risk reduction of HF readmission or all-cause mortality at 180 days

Improved quality of life and no increased risk of serious adverse events
NT-proBNP was used to inform safe and effective therapy
STRONG-HF video series
The importance of heart failure treatment
A diagnosis of heart failure can feel frightening and hopeless for patients. But optimal, guideline-directed medical therapy can dramatically reduce the risk of hospitalization or death from heart failure, and with good care, event- free survival can be extended by more than eight years.3 Watch this video to learn why heart failure should be treated with a sense of urgency.
Understanding the STRONG-HF clinical study
As the first of its kind, the Safety, Tolerability and Efficacy of Rapid Optimization, Helped by NT-proBNP Testing, of Heart Failure Therapies (STRONG-HF) clinical study assessed the safety and efficacy of simultaneous and rapid up- titration of heart failure medications. The study included frequent NT-proBNP measurements and close follow-up in patients admitted to a hospital for acute heart failure and for 180 days post-discharge. The results ultimately showed that these therapies can be rapidly and safely up-titrated post-event, and that this optimized heart failure care protocol leads to improved outcomes and quality of life for patients. Learn more about the impact of this groundbreaking study in this video.
The key role of natriuretic peptides
The use of B-type natriuretic peptide (BNP) and its N-terminal fragment (NT-proBNP) — which are released into the blood when the heart wall is stretched — as biomarkers in the heart failure space has evolved over the past two decades. The STRONG-HF clinical study showed that NT-proBNP, used as an objective measure of heart failure-related changes in conjunction with frequent visits, decreased following the implementation of an optimized heart failure protocol. Watch this video to learn more about this simple blood test and its use in managing patients living with heart failure.
Implementing STRONG-HF
The results of STRONG-HF have the potential to usher in a culture shift in heart failure management by demonstrating the safety and efficacy of rapid-sequence, simultaneous GDMT initiation. Watch this video to hear from four experts on why they are confident in the positive impact this can have for patients living with heart failure, and how implementing this protocol can also benefit clinicians and institutions.
References
- NIH.gov. Last accessed February 9, 2024.
- Mebazaa A, et al. Lancet. 2022;400(10367):1938-1952
- Vaduganathan M, et al., Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: a comparative analysis of three randomised controlled trials. Lancet. 2020 Jul 11;396(10244):121-128. doi: 10.1016/S0140-6736(20)30748-0.
In the United States, around 37 million people have diabetes, with an additional 1.4 million new diagnoses annually.1 For people who have diabetes there’s an additional health risk.
The correlation between diabetes and heart failure is significant, leading to a higher prevalence of heart failure in individuals with diabetes.2 Notably, those with diabetes and heart failure often face a more challenging prognosis compared to those who do not have diabetes but live with heart failure. This includes elevated rates of hospitalization and mortality, highlighting the critical need for proactive measures.2
Which is why, for the first time, heart failure testing recommendations have been included in the 2024 update of the American Diabetes Association’s (ADA) Standards of Care in Diabetes.3
These guidelines on diabetes and heart failure advocate for routine screening of heart failure in people living with diabetes. The objective is to detect the early onset of heart failure, enabling timely interventions to prevent its progression and improve outcomes.4
Benefits of cardiac biomarkers for people with diabetes
The ADA recommends annual NT-proBNP or BNP biomarker testing for individuals with diabetes to improve heart failure risk prediction, early diagnosis, and clinical decision-making. Cardiac biomarkers serve as the most sensitive tools for detecting ventricular wall stress, injury, and heart remodeling associated with heart failure development and contribute to improved patient outcomes. Despite the ADA's endorsement of both biomarker types, a laboratory-based NT-proBNP assay offers greater advantages.5
Why use n-terminal pro b-type natriuretic peptide (NT-proBNP)?
Even though the ADA supports the use of NT-proBNP or b-type natriuretic peptide (BNP), there are greater advantages to using a laboratory-based NT-proBNP assay:
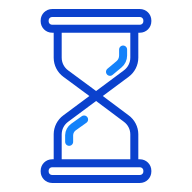
Half-life of approximately 60-120 minutes in the circulation5
Longer half-life of NT-proBNP contributes to levels which are six times higher than BNP although they are released in equimolar concentrations in the circulation from the same pro-hormone.
Lower biological variability than BNP.6
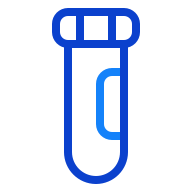
Stability of up to 3 days in the sample container or tube
Greater stability enables lab flexibility for outreach programs requiring transportation and add-on testing.

Standardized results and high NPV
Uniform results across instrument platforms allow for standardization throughout a system. High NPV supports clinicians in ruling out heart failure.

No ARNi interference
BNP levels will appear elevated in patients taking an Angiotensin Receptor Neprilysin Inhibitor (ARNi) such as Entresto®, which could lead to erroneous treatment.
Improving outcomes for people living with diabetes and heart failure
Early diagnosis of heart failure in individuals with diabetes holds the potential to initiate targeted treatment, prevent disease progression and mitigate adverse outcomes. The widespread adoption of annual cardiac biomarker monitoring in diabetes care can enhance the timely identification and diagnosis of heart failure at an earlier stage. This approach not only allows for improved treatment strategies but also contributes to delaying disease progression, offering the prospect of an enhanced quality of life for people living with diabetes for an extended period.

Diabetes and Heart Failure: How annual cardiac biomarker testing can help improve patient outcomes
Proactively measuring natriuretic peptide levels in people with diabetes represents a new heart failure diagnostic protocol.

American Diabetes Association updates guidelines concerning heart failure
This marks the first time use of cardiac biomarkers for heart failure risk prediction have been included.
References
- CDC.gov. Last accessed February 9, 2024
- Rosano, G. M., Vitale, C., & Seferovic, P. (2017). Heart Failure in Patients with Diabetes Mellitus. Cardiac failure review, 3(1), 52–55.
- American Diabetes Association Professional Practice Committee; Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 47 (Supplement_1).
- American Diabetes Association Professional Practice Committee; 10. Cardiovascular Disease and Risk Management: Standards of Care in Diabetes—2024. Diabetes Care 1 January 2024; 47 (Supplement_1): S179–S218.
- Gaggin, HK, Januzzi JL, The past, the present, and the future of natriuretic peptides in the diagnosis of heart failure, European Heart Journal Supplements, 2018;20(G)11–20.
- Tsutsui H, Albert NM, Coats AJS, et al. Natriuretic peptides: role in the diagnosis and management of heart failure: a scientific statement from the Heart Failure Association of the European Society of Cardiology, Heart Failure Society of America and Japanese Heart Failure Society. European Journal of Heart Failure, 2023;25(5):616-631.

