VENTANA PD-L1 (SP142) Assay Testing Sites
VENTANA PD-L1(SP142) Assay
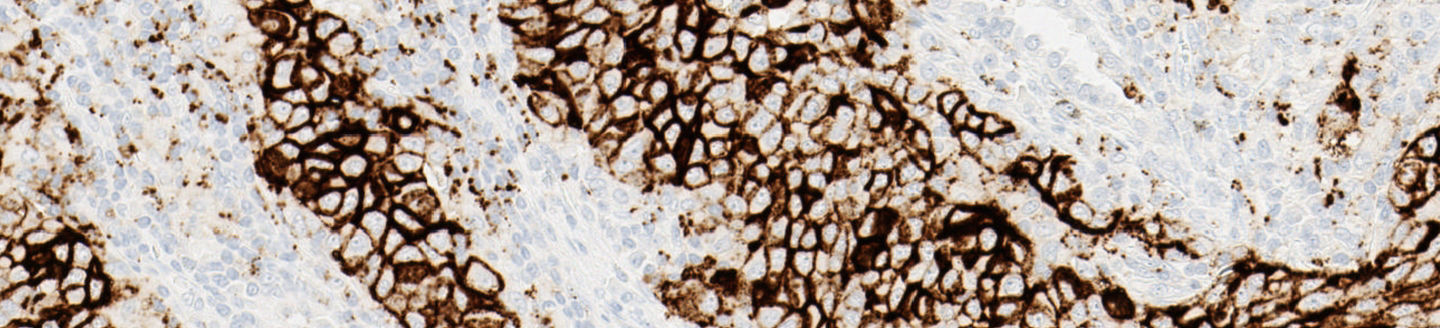
The VENTANA PD-L1 (SP142) Assay:
- FDA approved to identify NSCLC patients eligible for TECENTRIQ®
- Informative for the clinician of a patient’s potential overall survival
- Novel scoring algorithm using PD-L1 staining in both TC and IC
- Designed to enhance visual contrast of immune cell staining within the tumor microenvironment
The PD-L1 (SP142) Assay gives you the confidence to guide immunotherapy decisions in NSCLC.
Aurora Diagnostics LMC Pathology Services
7455 W. Washington Avenue, Suite 301
Las Vegas, NV 89128
Phone: 877-LMC-LABS
Phone: 702-732-3441
Fax: 702-732-2310
www.auroradxlmc.com
Integrated Oncology
www.integratedoncology.com
Arizona
Phone: 800-710-1800
Fax: 800-481-4151
New York
Phone: 800-447-5861
Fax: 212-258-2143
Tennesee
Phone: 800-874-8532
Fax: 615-370-8074
Laboratory Corporation of America
www.labcorp.com
Phone: 800-345-GENE (4363)
Fax: 888-287-8199
1904 Alexander Drive
Research Triangle Park, NC 27709
NeoGenomics Laboratories
31 Columbia
Aliso Viejo, CA 92656
Phone: 888-443-3311
www.neogenomics.com
PhenoPath Laboratories
551 North 34th St, Suite 100
Seattle WA 98103
Phone: 206-374-9000
Fax: 206-374-9009
www.phenopath.com/
Providence Regional Laboratory Services
Test Code: PD-L1
4400 NE Halsey St
Portland, OR 97213
Phone: 503-893-7777
www.providence.org
Quest Diagnostics Nichols Institute
33608 Ortega Highway
San Juan Capistrano, CA 92690 USA
Phone: 949-728-4879
Fax: 949-728-4992
www.nicholsinstitute.com
Tempus Labs, Inc.
600 W. Chicago Ave. Suite 510,
Chicago IL 60654
Phone: 833.514.4187
www.tempus.com
Thomas Jefferson University Hospitals, Inc.
Test Code: PDL1 UC
132 S. 10th Street, Room 283 Main Bldg.
Philadelphia, PA 19107
Phone: 215-955-6352
www.jefferson.edu
If you would like your lab featured on this list, please opt in here.