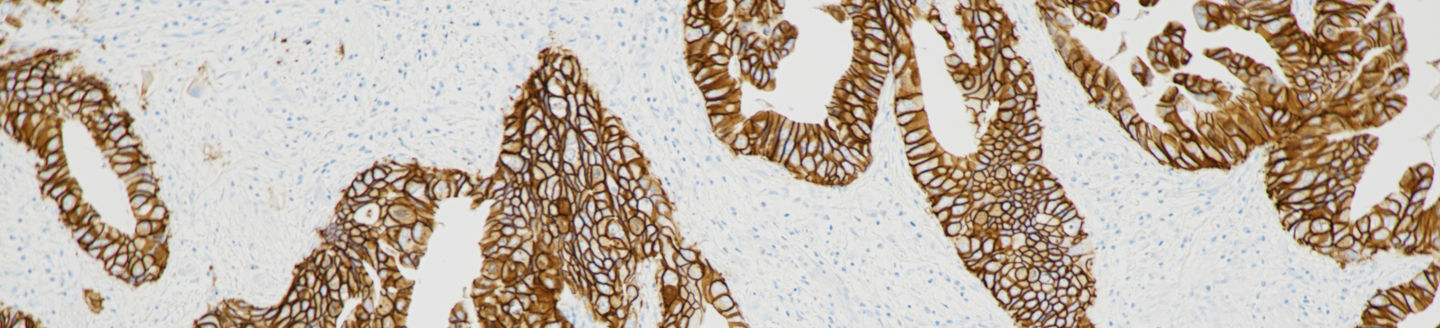
The PATHWAY HER2 (4B5) test helps identify patients with previously treated, unresectable or metastatic HER2-positive biliary tract cancer (BTC) who may be eligible for treatment with ZIIHERA.
There are currently very few options for BTC patients as most cases are at an advanced stage at the time of diagnosis.
This approval represents a new indication for Roche’s existing PATHWAY HER2 (4B5) test and expands its clinical utility by broadening the scope of patients who are eligible for HER2-targeted therapies.
TUCSON and INDIANAPOLIS; November 25, 2024 — Roche (SIX: RO, ROG; OTCQX: RHHBY) today announced U.S. Food and Drug Administration (FDA) approval of a label expansion into biliary tract cancer (BTC) for the PATHWAY® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody* test. This test is now the first and only FDA-approved companion diagnostic to aid in the assessment of HER2-positive status to identify BTC patients who are eligible for treatment with Jazz Pharmaceuticals’ ZIIHERA® (zanidatamab-hrii).
HER2 is a receptor protein expressed in a variety of cancers and serves as a predictive biomarker to help determine if a patient will respond to HER2-targeted therapy.1 No approved and validated HER2 test existed to identify eligible BTC patients until the approval of this expanded label for the PATHWAY HER2 (4B5) test.
This test is a step forward in furthering access to personalized medicine,” said Jill German, Head of Pathology Lab at Roche Diagnostics. “The prognosis for patients diagnosed with BTC is poor, as very few treatment options exist. Now, these patients have access to the first standardized test that could make them eligible for targeted therapy, potentially improving clinical outcomes.
ZIIHERA is the first FDA-approved treatment for adults with previously-treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer.
BTC accounts for 3% of all gastrointestinal cancers in the U.S.2,3 Prognosis is poor because of a lack of adequate early detection tools, challenging anatomical access, aggressive tumor biology and only modest benefit from systemic treatments.4 With most cases diagnosed at an advanced stage,5 the five-year overall survival rate for all BTC cases is 19% for disease that is localized to the original tumor site, and just 3% for cancer that has spread to other areas.6
About the PATHWAY® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody
The PATHWAY anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody delivers timely, clear and reliable results, enabling therapeutic decisions that can lead to better outcomes for patients. Already indicated as an aid to identify breast cancer patients eligible for HER2-targeted treatment with Herceptin®, KADCYLA® or ENHERTU®,7 the test is used in combination with the fully automated VENTANA BenchMark slide staining instrument. The introduction of the new indication for BTC represents a significant expansion of the test’s clinical utility. The assay forms an important part of Roche’s comprehensive gastrointestinal cancer solutions portfolio, which is aimed at driving diagnostic certainty for life-changing decisions in cancer care.
The assay standardizes all immunohistochemistry (IHC) processes from baking through staining, and reduces the possibility of human error.8 It also minimizes inherent variability resulting from individual reagent dilution and other processes found in manual and semi-automated IHC methods. The Roche HER2 (4B5) clone achieves consistently high-proficiency assessment scores compared to other clones9 and demonstrates high concordance with HER2 FISH,10,11 empowering laboratories to employ the most widely adopted and reliable HER2 IHC primary antibody.
For more information about the portfolio, please visit the Roche Diagnostics Pathology Lab companion diagnostics page.
About Roche
Founded in 1896 in Basel, Switzerland, as one of the first industrial manufacturers of branded medicines, Roche has grown into the world’s largest biotechnology company and the global leader in in-vitro diagnostics. The company pursues scientific excellence to discover and develop medicines and diagnostics for improving and saving the lives of people around the world. We are a pioneer in personalized healthcare and want to further transform how healthcare is delivered to have an even greater impact. To provide the best care for each person we partner with many stakeholders and combine our strengths in Diagnostics and Pharma with data insights from the clinical practice.
For over 125 years, sustainability has been an integral part of Roche’s business. As a science-driven company, our greatest contribution to society is developing innovative medicines and diagnostics that help people live healthier lives. Roche is committed to the Science Based Targets initiative and the Sustainable Markets Initiative to achieve net zero by 2045.
Genentech, in the United States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical, Japan.
For more information, please visit www.roche.com.
* Hereafter referred to as PATHWAY HER2 (4B5) test.
All trademarks used or mentioned in this release are protected by law.