What is dual-stain triage?
Dual stain identifies abnormal cells through biomarker staining of cervical cells collected during routine cervical cancer screening. It uses two biomarkers – p16 and Ki-67 – that, when found together, signal changes at the cellular level that indicate a transforming HPV infection.
The biomarkers provide an objective interpretation of the drivers behind transforming HPV infection, the co-expression of halting and progressive cell division. This provides greater risk and disease stratification when screening for cervical cancer.
How does the science behind dual-stain technology work?
p16 is a biomarker that occurs in normal cells and signifies cell cycle arrest. If you think of it like a stoplight, it tells you to stop. It’s indicated by brown staining in the cytoplasm.
Ki-67 is a biomarker that occurs in normal cells and signifies cell division. It’s indicated by the red stain in the nucleus. When the two biomarkers – p16 and Ki-67 – are both in the cell at the same time, it's like a traffic light where the red and green lights are on at the same time and it's like, Whoa, do I stop or do I go? So you know there is something wrong.
The HPV is in the cell, integrating into the DNA and causing transformation before you actually see it in the morphology of the cell. Cytology is subjective but dual stain is a more objective, biomarker-based assay, and you interpret it completely independent of cytology.
What does this information tell us?
Dual stain tells us what a patient's cells are doing in response to HPV infection, independent of their HPV genotype. It tells us whether or not the high-risk HPV E6/E7 are affecting a patient's tumor suppressor proteins, and it tells us if a patient's cells are undergoing oncogenic transformation.
How does this information impact risk?
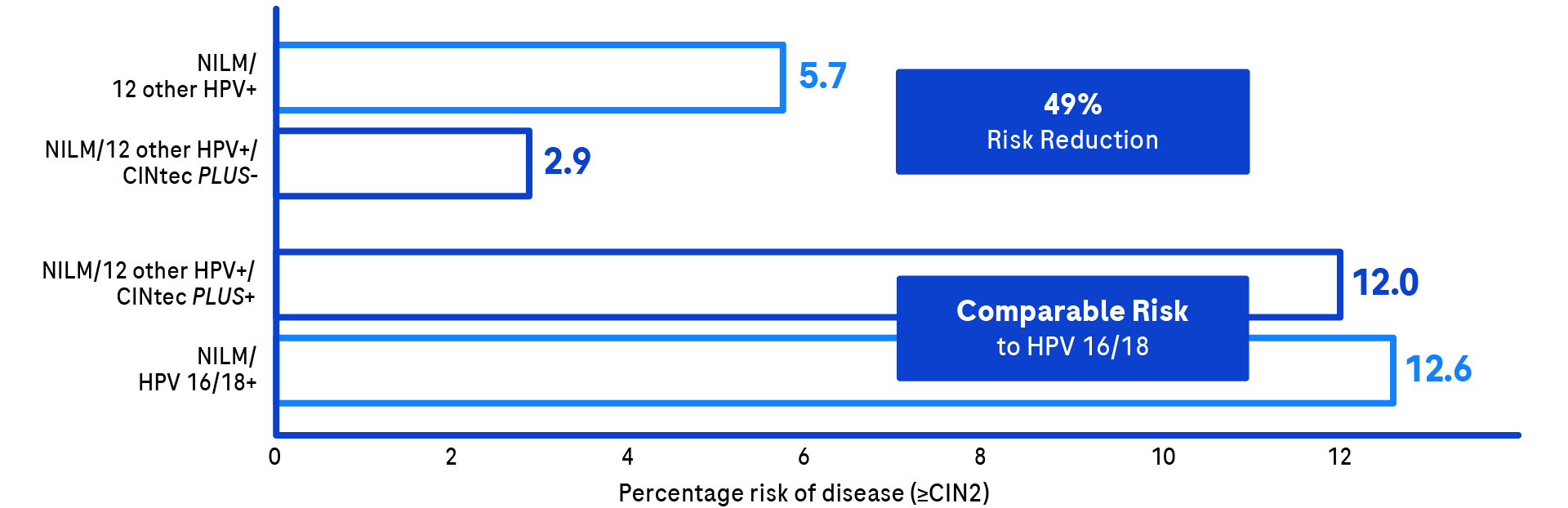
Under the current guidelines, most patients with discrepant co-testing repeat in 12 months. We know if we send someone away who has NILM with 12 other HPV, she has a 5.7% chance of having a developing CIN2 or greater. If we do CINtec PLUS and send her away after a negative result, we know she has only a 2.9% chance of developing CIN2 or greater.
Also, if you look at the risk of having a known HPV-positive test for HPV 16 or 18, those are the ones we’re taking to colposcopy; their risk is 12.6%. When you have a normal 12-other HPV positive result with a positive CINtec PLUS, the risk is 12%. Because those risks are pretty equal, it makes sense that those people could benefit from colposcopy rather than coming back the next year.
What does the big picture of a dual-stain test mean?
The ATHENA trial showed us that one in four women who are HPV 16 positive will have cervical disease in three years. If we can find those people before they have major abnormalities on their cytology, we have done them a service because we have found them earlier and have a greater chance of preventing CIN3.
How should dual-stain testing be used?
When patients test positive with the cobas HPV test, that same sample can be used with CINtec PLUS, a biomarker test, to determine if the patient is progressing toward cervical cancer. In primary HPV screening, CINtec PLUS Cytology can be used as a triage test instead of Pap cytology.4 In co-testing, CINtec PLUS Cytology can be used as a triage test to resolve seemingly discrepant HPV positive/Pap normal screening results.