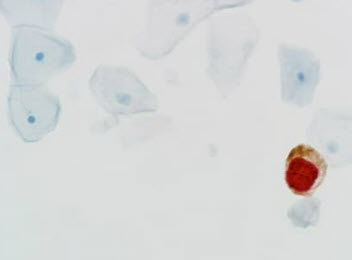
Cervical cancer, once a leading cause of death for women, has seen a significant reduction in the past four decades due to the widespread use of routine Pap tests and human papillomavirus (HPV) testing. Despite progress, more than 50% of all new cervical cancer cases are diagnosed in women who have never been screened or have not been screened in the last 5 years,¹ underscoring the importance of continuous vigilant screening. Annually, 13,820 people are diagnosed with cervical cancer in the U.S., resulting in 4,360 deaths.²
While Pap cytology has been in use since 1928,³ recent technological advances in HPV testing have provided clinicians with the ability to identify patients at higher risk of cervical cancer based on their HPV genotype.
Approximately 99.7% of cervical cancer cases are caused by HPV. While nearly 90% of HPV infections resolve without progressing to cancer, regular screening and diagnostic testing remain critical to identify individuals at risk of developing cervical cancer.4 Timely and informed testing is especially critical because more than half of women with abnormal cervical screening results will not return for subsequent testing, meaning cervical disease could be missed.5 Recognizing this challenge, Roche Diagnostics, committed to shaping a future with longer, healthier lives, offers a comprehensive FDA-approved Cervical Cancer Portfolio. Our innovative solutions aim to address screening limitations, enabling earlier diagnosis and treatment that prioritize patient well-being.
Advances in cervical cancer screening and triage
Our cobas® HPV test can detect the HPV genotypes that have shown the highest risk of cancer development, including HPV-16 and HPV-18 and 12 other pooled genotypes. And, women and people with a cervix can collect their own vaginal sample in a healthcare setting to make HPV testing for cervical screening more comfortable and accessible. In December 2024, the U.S. Preventive Services Task Force, also known as USPSTF, concluded that “Self-collection of HPV test for screening has similar accuracy to clinician-collected tests and is associated with increased screening in underscreened individuals and in historically underscreened populations” in their updated Draft Recommendation for Cervical Cancer: Screening.
Our dual-stain cytology triage technology can detect the transformation of HPV-infected cells into precancer. For example, in a dual-stain biomarker triage test, p16 is a biomarker that occurs in normal cells and signifies cell cycle arrest. Ki-67 is also a biomarker that occurs in normal cells and signifies cell cycle division. A new test can identify when both biomarkers are present at the same time, indicating that HPV is disrupting the cell cycle and the cell has started to transform.
The American Society of Colposcopy and Cervical Pathology, also known as ASCCP, took a historic step in March 2024 by including dual-stain cytology technology in its Enduring Guidelines to help clinicians to triage patients who are positive for the types of HPV that have higher risks of developing cervical cancer.
Early detection significantly improves the five-year survival rate, which is 91% for women with invasive cervical cancer when identified early.⁶
References
- pubmed.ncbi.nlm.nih.gov/21263352/. Accessed 12/2025.
- Cancer.gov. Accessed 12/2025.
- Pubmed.gov. Accessed 12/2025.
- pmc.ncbi.nlm.nih.gov/articles/PMC7062568/. Accessed 12/2025.
- jamanetwork.com/journals/jamanetworkopen/fullarticle/2829357. Accessed 12/2025.
- Cancer.gov. Accessed 12/2025.

Only the Roche Cervical Cancer Portfolio covers the entire spectrum of screening, triage and diagnostic testing
Cervical Pap smear tests have evolved to detect potentially precancerous and cancerous processes in the cervix. While Pap cytology has played a crucial role, recent technological advancements include HPV testing, providing better insights into patients' HPV genotypes and identifying individuals at higher risk of developing cervical cancer.
Featuring molecular and biomarker-based tests, our portfolio offers enhanced diagnostic certainty in cervical cancer screening. These advanced tests provide guidance to clinicians and women at every stage of the screening process, eliminating the ambiguity associated with current testing approaches.
Adapting to evolving medical guidelines, the Roche Cervical Cancer Portfolio accommodates various approaches, including co-testing, human papillomavirus (HPV) primary screening, HPV self-collection for primary screening and dual-stain cytology triage technology. This versatility ensures that the Portfolio remains at the forefront of supporting current and emerging best practices in cervical cancer screening and diagnosis.
cobas® HPV test supports clinicians’ screening methods
A screening tool exists that can stratify a woman's risk for cervical cancer. The cobas® HPV test is the first HPV test approved for all three screening options and collection vials, Thinprep and Surepath*.
The cobas® HPV test objectively identifies women at risk and improves the detection of high-grade disease in a single round of screening, utilizing molecular diagnostic testing for the presence of HPV.
The cobas® HPV test is the cervical cancer screening test approved for ASC-US reflex, co-testing, and primary screening, as well as for use with both types of specimen collection vials* giving healthcare providers the flexibility to choose the best screening method for patients.3,4
The cobas® HPV test is one of the first HPV self-collection solutions approved in the U.S. In a healthcare setting, women collect their own vaginal samples, which are then sent to a laboratory for analysis with Roche’s cobas® molecular instrument.
*Surepath is only available on the cobas 4800.

Screen with confidence
The cobas® HPV test is specifically designed to enhance screening accuracy by minimizing the risk of false negatives across all high-risk HPV genotypes. Providing three results in one test, it covers the 14 types of high-risk HPV genotypes associated with cervical cancer, including specific results for HPV 16, HPV 18, and 12 other pooled genotypes. This comprehensive approach furnishes essential information for critical clinical decisions.
To prevent false-negative results, the test monitors the presence of human cells and confirms the completion of reactions. It also demonstrates no cross-reactivity with low-risk HPV genotypes, ensuring that a positive result is truly positive. The reliance on DNA-based testing is backed by 25 years of data, derived from millions of women, supporting the importance of DNA in viral infection replication.⁵

CINtec PLUS is a dual-stain triage cytology test
Cervical Pap smear tests have evolved to detect potentially precancerous and cancerous processes in the cervix. While Pap cytology has historically played a crucial role, recent technological advances, such as HPV testing, can provide additional insights into early disease indicators that Pap tests cannot detect. Additionally, HPV testing can identify individuals at higher risk of developing cervical cancer.
The emergence of dual-stain technology allows for the detection of HPV-infected cell transformation into precancer. Early detection significantly improves the five-year survival rate for invasive cervical cancer, reaching 92%.⁶ The CINtec® PLUS Cytology test was introduced in 2020 and remains the only FDA-approved dual-stain biomarker triage test available today.
Detect changes at the cellular level
The CINtec PLUS Cytology test is the only a dual-stain biomarker-based cytology test FDA-approved for HPV primary screening with clinician-collected cervical samples and HPV-positive and Pap-negative co-testing results. This assay identifies abnormal cells in cervical cytology samples using p16 and Ki-67 biomarkers to detect transforming HPV infections.⁷
Biomarkers provide an objective interpretation of the drivers behind transforming HPV infection: simultaneous co-expression of halting and progressive cell division. Co-expression of the two biomarkers, p16 and Ki-67, indicates that HPV is disrupting the cell cycle and has started cell transformation. This additional information further stratifies risk and objectively identifies evidence of cervical disease to enable a more informed understanding of patient's risk of developing cervical cancer earlier in the screening process when screening for cervical cancer.

CINtec PLUS Cytology is approved as a triage/reflex with the cobas HPV Test:1
Triage testing following HPV primary screening for HPV positive results in women ages 25-65

* For HPV16/18+ use as additional information in conjunction with the physician’s assessment of patient screening history, other risk factors, and professional guidelines to guide patient management.
Source: Adapted from CINtec PLUS Cytology package insert; Refer to the CINtec PLUS Cytology package insert for the FDA approved intended use.
Reflex testing following discrepant co-testing results in women ages 30-65

* For HPV16/18+ use as additional information in conjunction with the physician’s assessment of patient screening history, other risk factors, and professional guidelines to guide patient management.
Source: Adapted from CINtec PLUS Cytology package insert; Refer to the CINtec PLUS Cytology package insert for the FDA approved intended use.
In April of 2024, recommendations for the use of dual-stain (DS) cytology with CINtec PLUS Cytology (Roche) were developed by the American Society for Colposcopy and Cervical Pathology (ASCCP) Enduring Consensus Cervical Cancer Screening and Management Guidelines Committee.1 These recommendations were based on clinical action thresholds that were developed for the ASCCP 2019 Risk- Based Management Consensus Guidelines.
According to the updated guidelines, dual-stain cytology may be used in conjunction with certain HPV/Pap co-testing results and/or as a triage test for certain HPV primary screening results.19
CINtec Histology can diagnose cervical cancer
CINtec® Histology is the only FDA-cleared p16 biomarker technology that can help pathologists evaluate cervical biopsy specimens that can confirm the presence of precancerous cervical lesions.⁸
For patients in need of a colposcopy, CINtec Histology provides a more objective interpretation of cervical biopsies and, in one trial, decreased potentially false negative results by 43%.⁷
Our test detected 85 out of 100 biopsies with the disease compared to 73 out of 100 with hematoxylin and eosin stains.⁷

IMPACT Trial
Recognizing that cervical cancer trials need to be more inclusive and representative, especially for populations most at risk for cervical cancer, our trial deliberately included diverse patient segments, with 21% Black, 24% Hispanic-Latina, and 0.3% American Indian or Alaskan Native participants.² By being more inclusive, Roche aims to accurately reflect patient populations with higher incident rates of HPV, contributing to efforts to address health impacts in cervical cancer.
The IMPACT Trial design validated the clinical benefits of the cobas HPV Test, CINtec PLUS Cytology test and CINtec Histology.¹ In the trial, CINtec PLUS Cytology detected cervical disease earlier in 7 out of 10 women who were already identified as HPV positive.⁷ CINtec® PLUS is a triage cytology test that helps stratify disease risk immediately, giving clinicians confidence when selecting the appropriate management for women at every level of risk.
References
- Centers for Disease Control. Last accessed January 2, 2024.
- Wright TC Jr, Stoler MH, Ranger-Moore J, Fang Q, Volkir P, Safaeian M, Ridder R. Clinical validation of p16/Ki-67 dual-stained cytology triage of HPV-positive women: Results from the IMPACT trial. Int J Cancer. 2021 Sep 18. doi: 10.1002/ijc.33812. Online ahead of print.
- cobas® HPV test package insert.
- cobas® HPV Test for cobas® 6800/8000 package insert.
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, Broker TR, Stanley MA. The biology and life-cycle of human papillomaviruses. Vaccine. 2012 Nov 20;30 Suppl 5:F55-70. doi: 10.1016/j.vaccine.2012.06.083. PMID: 23199966.
- Cancer.net. January 2, 2024.
- CINtec® PLUS Cytology package insert.
- CINtec® Histology 510(k) package insert.

Every two hours in the U.S., a woman dies of cervical cancer, which is preventable.¹
Cervical cancer is one of the most preventable cancers today due to vaccination, screening and early treatment. Yet it is still responsible for thousands of deaths each year.²
Unlike most cancers, more than 99% of cervical cancer cases are caused by an infection – human papillomavirus (HPV).³
Four out of five women will be infected with HPV at some point in their lives.⁴ Most of the time, HPV infections clear naturally and do not cause cancer. While there are more than 200 different strains of HPV,⁵ there are 14 genotypes that are most commonly associated with cervical cancer.
Definitions
What is cervical cancer?
Cervical cancer is cancer of the cervix. The cervix is located at the lowest part of the uterus. A persistent or long-term infection of certain high-risk HPV types is the primary cause of cervical cancer. When found early or in the pre-cancer stage, treatment can be successful. Cervical cancer is highly preventable with vaccination and regular screening.⁶
What is HPV?
The HPV is the most common sexually transmitted infection. HPV is so common that nearly all sexually active adults, regardless of birth-assigned sex or gender identity, will have at least one HPV infection at some point in their lives. Most HPV infections have no signs or symptoms and are cleared by the body’s immune system.7
What is primary screening?
Primary HPV screening is when an HPV test is administered without another test.17
Is cervical screening covered by insurance?
Regular screenings for HPV – no cost for most under the Affordable Care Act – can lessen the chances of cervical cancer developing.18
The importance of cervical cancer screening
Screening is key to reducing the risk of developing cervical cancer. All women above age 21,16 or once sexually active, need cervical cancer screening, no matter their sexual orientation, relationship status or HPV vaccine status.⁹
There are two options healthcare providers use to screen: the Pap and HPV tests. The two screening options can be done together or independently, depending on age or medical history and the doctor’s recommendation.
Pap test
A Pap test looks for abnormal cells on the cervix that can indicate early signs of cancer.
The Pap test has been in use for around 80 years; it looks for abnormal cells growing on the cervix that can be early signs of cancer. A healthcare provider collects cell samples from the cervix and sends the sample to a laboratory for examination under a microscope by a trained professional. However, a normal Pap result does not always mean cancer-free: Up to one-third of cervical cancers occur in women with a normal Pap.10
HPV test
An HPV test detects high-risk human papillomavirus.
An HPV test detects the DNA of high-risk HPV: an infection that may lead to high-grade cervical disease or precancer. The sample needed for an HPV test is taken from the cervix by a healthcare provider in the same way as the Pap test. Women and people with a cervix also may have the option of taking their own vaginal sample in a healthcare setting to make HPV testing for cervical screening more comfortable and accessible. Samples taken by a clinician or by the women being screened are then sent to a lab to be tested for high-risk HPV on an automated instrument using molecular technology. HPV tests give an early, accurate look at cervical cancer risk.
The difference between the two screening tests is the Pap test looks for changes in cells before they develop into cancer.
The HPV test looks for high-risk HPV genotypes to better manage risk for cervical cancer. Receiving an HPV test can better predict one’s risk of cervical cancer.
Roche offers the cobas® HPV test. The test is used in cervical cancer screening to determine a woman's risk of precancer or cancer. The test provides individual results for high-risk HPV genotypes 16 and 18 and a combined result for 12 other high-risk genotypes.
Screening recommendation by age:16


Understanding HPV screening results
Testing positive for HPV is not a reflection of sexual behavior or lifestyle, and it can happen in a monogamous relationship, as the virus can go undetected for years. Most HPV infections have no signs or symptoms and are cleared by the body’s natural immune system.⁴ A positive result does not mean one will have or will develop cervical cancer, but it does mean there’s an increased risk.11
There are five potential combinations of cervical screening results:11
Pap test (cervical cytology) results
Normal Pap no cell changes were found on the cervix
There is a low chance of developing cervical pre-cancer or cancer; one should have another screening test in 3 years.
Pap unclear
An unclear pap is also known as ASC-US, atypical squamous cells of undetermined significance. Some cells on the cervix do not look normal, but the changes do not clearly suggest a precancerous lesion. Your doctor may recommend an HPV test to find out if the cell changes are related to HPV.
Pap abnormal cell changes were found on the cervix
The changes may be minor (low-grade) or moderate to severe (high-grade). This does not mean one has cervical cancer. Most of the time, minor changes go away on their own. However, more severe changes may result in cervical cancer if not treated. The doctor may suggest an HPV test or colposcopy to help determine the next steps.
HPV test results
HPV negative
There is no HPV infection, and the risk is low for developing cervical pre-cancer or cancer in the next 5 years.
HPV positive
There is a high-risk HPV infection and an increased risk of cervical pre-cancer or cancer. The doctor may suggest waiting a year before retesting to allow the body more time to clear the infection, request additional testing to see if the cells are changing, or recommend a colposcopy to help determine the next steps. It is important that one returns for a follow-up visit.
Receiving a positive HPV diagnosis
Most women who are HPV-positive will not develop cervical cancer.11
Triage typically occurs when women test positive for high-risk HPV genotypes. Common high-risk genotypes are HPV 16 and HPV 18, which account for 66% of cervical cancer.12 The other high-risk genotypes are: 31, 33, 45, 52 and 58.13
After receiving a positive result for a high-risk HPV genotype, there are different steps that can be taken.14
- Some healthcare providers may suggest waiting another year to see if the infection will clear on its own.
- A healthcare provider could request that the sample be sent for a triage test to know if the infection is progressing.
- With certain types of high-risk HPV, a healthcare provider may want to biopsy to see if pre-cancer or cancer is present.
Roche’s CINtec® PLUS Cytology is a dual-stain triage cytology test that helps stratify risk and objectively identify evidence of cervical disease immediately. The dual-stain test is run on the same cervical sample collected by a clinician during a routine screening visit. The results provide women better information about whether their HPV infection is transforming into pre-cancer without having to wait a year for follow-up. In addition, CINtec PLUS dual-stain cytology results provide the clinician the most appropriate management for their patients according to risk-based guidelines: immediate intervention or routine follow-up testing. This is especially crucial considering more than half of women will not return for follow-up testing.
Definitions
What is triage?
Triage refers to the process of sorting people based on whether one may benefit from an immediate intervention or can be allowed more time before follow-up or retesting. In the case of cervical cancer screening, it may be recommended that women who receive a positive high-risk HPV and/or abnormal Pap result are managed more closely with follow-up care or testing.15
How do HPV genotypes help?
Genotypes can help doctors manage risk for their patients. An HPV genotype result can indicate if a patient has a higher risk of developing cervical cancer. Common high-risk genotypes are HPV 16 and HPV 18.15
What is dual-stain?
Dual-stain identifies abnormal cells through biomarker staining of cervical cells collected during routine cervical-cancer screening. It uses two biomarkers – p16 and Ki-67 – that when found together, signal changes at the cellular level that indicate a transforming HPV infection.
Take action
Cervical cancer prevention begins by being proactive. Vaccination and regular screening are important because they have been shown to prevent cancer and save lives. Ensuring everyone has access to cervical cancer screening is key to eliminating the disease.
Every woman should feel encouraged to talk openly with their doctor regarding available screening options and the recommended frequency for cervical cancer screening. No one should feel shame or embarrassment about discussing topics related to HPV or cervical cancer. Promoting awareness, accessibility, and destigmatization, communities can work together to prioritize early detection and prevention to help women everywhere.
Disclaimer: This content is provided for educational and informational purposes only and does not constitute providing medical advice or professional services. The information provided should not be used for diagnosing or treating a health problem or disease, and those seeking personal medical advice should consult with a licensed physician. Always seek the advice of your doctor or another qualified health provider regarding a medical condition.
References
- Cervicalcanceraction.org. Last accessed August 2, 2024.
- Cancer.org. Last accessed August 2, 2024.
- Cdc.gov. Last accessed August 2, 2024.
- Cdc.gov. Last accessed August 2, 2024.
- Nih.gov. Last accessed August 2, 2024.
- Cancer.gov. Last accessed August 2, 2024.
- Cancer.gov. Last accessed August 2, 2024.
- Cancer.gov. Last accessed August 2, 2024.
- Ncbi.gov. Last accessed August 2, 2024.
- Ncbi.gov. Last accessed August 2, 2024.
- Cdc.gov. Last accessed August 2, 2024.
- Cdc.gov. Last accessed August 2, 2024.
- Cancer.gov. Last accessed August 2, 2024.
- Cancer.gov. Last accessed August 2, 2024.
- Ncbi.gov. Last accessed August 2, 2024.
- Uspreventiveservicestaskforce.org. Last accessed August 2, 2024.
- Cancer.gov. Last accessed August 2, 2024.
- Hrsa.gov. Last accessed August 2, 2024.
- ASCCP.org. Last accessed August 2, 2024.
Featured products
Benefits of Roche diagnostic solutions for managing cervical cancer
A leader in cervical cancer diagnosis, with trusted experience
A focus on the science of HPV has led to an evolution in cervical cancer screening strategies to better stratify patient risk, and improve outcomes. With the best scientists, and continuous commitment to the elimination of cervical cancer, we have built our organization around always striving to meet our customers’ needs and improve patient care.
Our holistic solution for cervical cancer screening, triage, and diagnosis is the most broadly recommended in guidelines to answer three questions that change women's lives and their communities:
- Is she at risk?
- Does she have evidence of cervical disease?
- Am I certain of her diagnosis?
Roche's portfolio, now offering options for self-collection, reaches across geographies and cultures, so no one ever has to die from a preventable disease.
Roche has a long history as a scientific leader and innovator, driving cervical screening strategies and a change from the status quo, because we believe women deserve better. Roche continues to make significant investment in the cervical screening portfolio and in new product development, backed by strong clinical study evidence. In cervical cancer screening, with the goal of elimination in mind, we also recognize that digital innovations can bring efficiencies and scale to population-based programs, and with attention that also zeroes in at the patient level, we help to ensure that no one is left behind.
We launched the very first:
- FDA approved cobas® HPV test for primary cervical screening
- FDA approved dual-stain immunocytologic CINtec® PLUS Cytology triage test
- FDA cleared diagnostic p16 biomarker immunohistochemistry test (CINtec® Histology)
Roche remains a leader in in vitro diagnostics (IVD), trusted by customers around the globe, with over 29 billion tests completed in 2022.1
References:
- F. Hoffmann-La Roche Ltd. Annual Report 2022 [Internet; cited 2024 July 3]. Available from: https://assets.cwp.roche.com/f/126832/x/7cd4e2ba4c/ar22e.pdf.
- F. Hoffmann-La Roche Ltd. cobas HPV Qualitative nucleic acid test for use on the cobas 5800/6800/8800 Systems Method Sheet. (v2.0). 2024.
Reaching more patients with a uniquely solutions-oriented, collaborative approach
We work closely with labs to deliver high-impact solutions that meet their needs, the needs of the clinicians ordering the tests, and population screening program requirements for patient database management. Our portfolio options are flexible to meet a guidelines-based patient care approach, with shared access across multiple site locations, serving key stakeholders within the cervical screening ecosystem, including payers or policy makers.
With our ability for technical and healthcare consultancy, our support ranges from finding an optimal configuration to improving operational efficiency.
Strong medical value across the continuum of care
For cervical cancer screening, we're paving the way for a new standard of care based on our understanding of HPV’s role in disease progression and how early HPV detection and treatment can prevent progression to cervical cancer. Our cutting-edge automation and integration streamline workflows, increase throughput, and accelerate turnaround times that ensure rapid and accurate results so patients know now if they are at increased risk.1
Our triage options for women with a positive high-risk HPV result further improve disease management decisions. Dual-stain biomarker technology, an immunocytologic triage test, can be performed using the same clinician-collected cervical sample for HPV primary screening, meaning no additional patient exam is needed, and there is less potential loss to follow-up.
Finding and treating disease early helps drive better outcomes. Knowing whether to take clinical action immediately, or allow a woman’s body more time to clear the virus that causes cervical disease can be reassuring to clinicians and their patients.
Our platform options provide the flexibility to scale throughput and testing capabilities to increase value and access to new market opportunities, without requiring extensive training or subjective, technical expertise. This frees up skilled staff to dedicate more time to high-value tasks and collaborate with clinicians to improve patient care. Our digital innovations remove the burden of data entry, which minimizes the risk of human error or paper-based analytics and program population study reviews.
Our comprehensive menu of assays and solutions spans nearly the entire spectrum of routine testing to better stratify risk for disease – along the continuum of screening, triage and diagnosis.
References:
- F. Hoffmann-La Roche Ltd. Annual Report 2022 [Internet; cited 2024 July 3]. Available from: https://assets.cwp.roche.com/f/126832/x/7cd4e2ba4c/ar22e.pdf.
- F. Hoffmann-La Roche Ltd. cobas HPV Qualitative nucleic acid test for use on the cobas 5800/6800/8800 Systems Method Sheet. (v2.0). 2024.
Explore more
References:
- F. Hoffmann-La Roche Ltd. Annual Report 2022 [Internet; cited 2024 July 3]. Available from: https://assets.cwp.roche.com/f/126832/x/7cd4e2ba4c/ar22e.pdf.
- F. Hoffmann-La Roche Ltd. cobas HPV Qualitative nucleic acid test for use on the cobas 5800/6800/8800 Systems Method Sheet. (v2.0). 2024.